Skrott Poster
advertisement
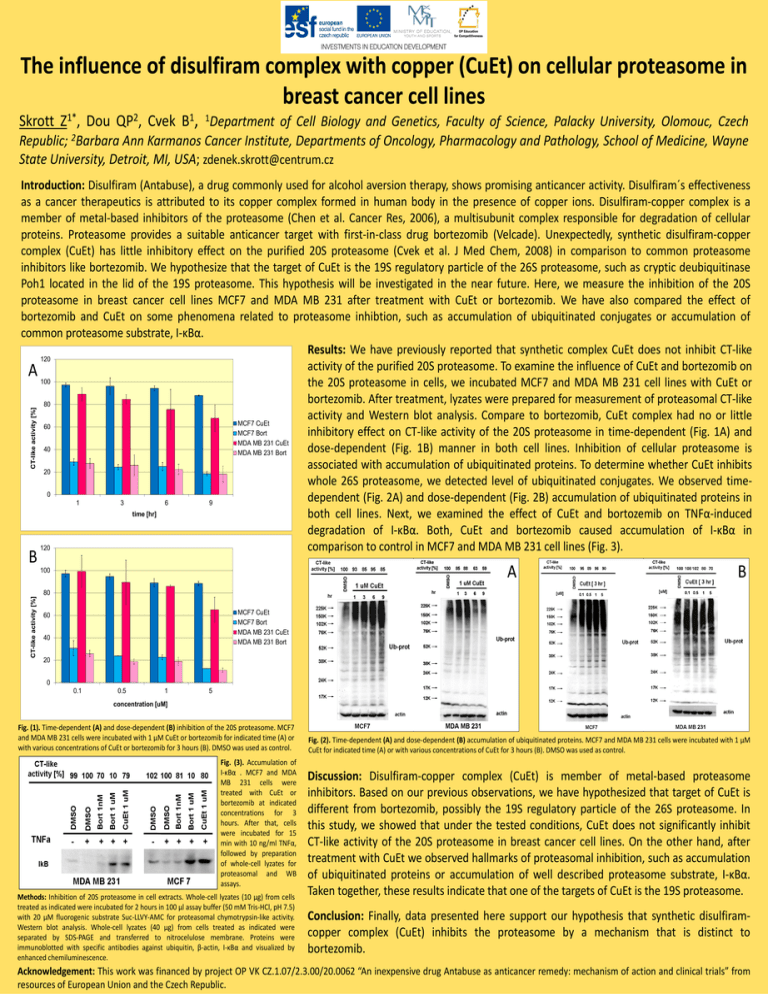
The influence of disulfiram complex with copper (CuEt) on cellular proteasome in breast cancer cell lines Skrott Z1*, Dou QP2, Cvek B1, 1Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Olomouc, Czech Republic; 2Barbara Ann Karmanos Cancer Institute, Departments of Oncology, Pharmacology and Pathology, School of Medicine, Wayne State University, Detroit, MI, USA; zdenek.skrott@centrum.cz CT-like activity [%] Introduction: Disulfiram (Antabuse), a drug commonly used for alcohol aversion therapy, shows promising anticancer activity. Disulfiram´s effectiveness as a cancer therapeutics is attributed to its copper complex formed in human body in the presence of copper ions. Disulfiram-copper complex is a member of metal-based inhibitors of the proteasome (Chen et al. Cancer Res, 2006), a multisubunit complex responsible for degradation of cellular proteins. Proteasome provides a suitable anticancer target with first-in-class drug bortezomib (Velcade). Unexpectedly, synthetic disulfiram-copper complex (CuEt) has little inhibitory effect on the purified 20S proteasome (Cvek et al. J Med Chem, 2008) in comparison to common proteasome inhibitors like bortezomib. We hypothesize that the target of CuEt is the 19S regulatory particle of the 26S proteasome, such as cryptic deubiquitinase Poh1 located in the lid of the 19S proteasome. This hypothesis will be investigated in the near future. Here, we measure the inhibition of the 20S proteasome in breast cancer cell lines MCF7 and MDA MB 231 after treatment with CuEt or bortezomib. We have also compared the effect of bortezomib and CuEt on some phenomena related to proteasome inhibtion, such as accumulation of ubiquitinated conjugates or accumulation of common proteasome substrate, I-κBα. Results: We have previously reported that synthetic complex CuEt does not inhibit CT-like 120 activity of the purified 20S proteasome. To examine the influence of CuEt and bortezomib on A 100 the 20S proteasome in cells, we incubated MCF7 and MDA MB 231 cell lines with CuEt or bortezomib. After treatment, lyzates were prepared for measurement of proteasomal CT-like 80 activity and Western blot analysis. Compare to bortezomib, CuEt complex had no or little MCF7 CuEt 60 MCF7 Bort inhibitory effect on CT-like activity of the 20S proteasome in time-dependent (Fig. 1A) and MDA MB 231 CuEt 40 dose-dependent (Fig. 1B) manner in both cell lines. Inhibition of cellular proteasome is MDA MB 231 Bort associated with accumulation of ubiquitinated proteins. To determine whether CuEt inhibits 20 whole 26S proteasome, we detected level of ubiquitinated conjugates. We observed time0 dependent (Fig. 2A) and dose-dependent (Fig. 2B) accumulation of ubiquitinated proteins in 1 3 6 9 time [hr] both cell lines. Next, we examined the effect of CuEt and bortozemib on TNFα-induced degradation of I-κBα. Both, CuEt and bortezomib caused accumulation of I-κBα in 120 comparison to control in MCF7 and MDA MB 231 cell lines (Fig. 3). B A CT-like activity [%] 100 B 80 MCF7 CuEt MCF7 Bort MDA MB 231 CuEt MDA MB 231 Bort 60 40 20 0 0.1 0.5 1 5 concentration [uM] Fig. (1). Time-dependent (A) and dose-dependent (B) inhibition of the 20S proteasome. MCF7 and MDA MB 231 cells were incubated with 1 μM CuEt or bortezomib for indicated time (A) or with various concentrations of CuEt or bortezomib for 3 hours (B). DMSO was used as control. Fig. (3). Accumulation of I-κBα . MCF7 and MDA MB 231 cells were treated with CuEt or bortezomib at indicated concentrations for 3 hours. After that, cells were incubated for 15 min with 10 ng/ml TNFα, followed by preparation of whole-cell lyzates for proteasomal and WB assays. Methods: Inhibition of 20S proteasome in cell extracts. Whole-cell lyzates (10 μg) from cells treated as indicated were incubated for 2 hours in 100 μl assay buffer (50 mM Tris-HCl, pH 7.5) with 20 μM fluorogenic substrate Suc-LLVY-AMC for proteasomal chymotrypsin-like activity. Western blot analysis. Whole-cell lyzates (40 μg) from cells treated as indicated were separated by SDS-PAGE and transferred to nitrocelulose membrane. Proteins were immunoblotted with specific antibodies against ubiquitin, β-actin, I-κBα and visualized by enhanced chemiluminescence. Fig. (2). Time-dependent (A) and dose-dependent (B) accumulation of ubiquitinated proteins. MCF7 and MDA MB 231 cells were incubated with 1 μM CuEt for indicated time (A) or with various concentrations of CuEt for 3 hours (B). DMSO was used as control. Discussion: Disulfiram-copper complex (CuEt) is member of metal-based proteasome inhibitors. Based on our previous observations, we have hypothesized that target of CuEt is different from bortezomib, possibly the 19S regulatory particle of the 26S proteasome. In this study, we showed that under the tested conditions, CuEt does not significantly inhibit CT-like activity of the 20S proteasome in breast cancer cell lines. On the other hand, after treatment with CuEt we observed hallmarks of proteasomal inhibition, such as accumulation of ubiquitinated proteins or accumulation of well described proteasome substrate, I-κBα. Taken together, these results indicate that one of the targets of CuEt is the 19S proteasome. Conclusion: Finally, data presented here support our hypothesis that synthetic disulfiramcopper complex (CuEt) inhibits the proteasome by a mechanism that is distinct to bortezomib. Acknowledgement: This work was financed by project OP VK CZ.1.07/2.3.00/20.0062 “An inexpensive drug Antabuse as anticancer remedy: mechanism of action and clinical trials” from resources of European Union and the Czech Republic.