Formula Mass and Percent Composition Practice
advertisement
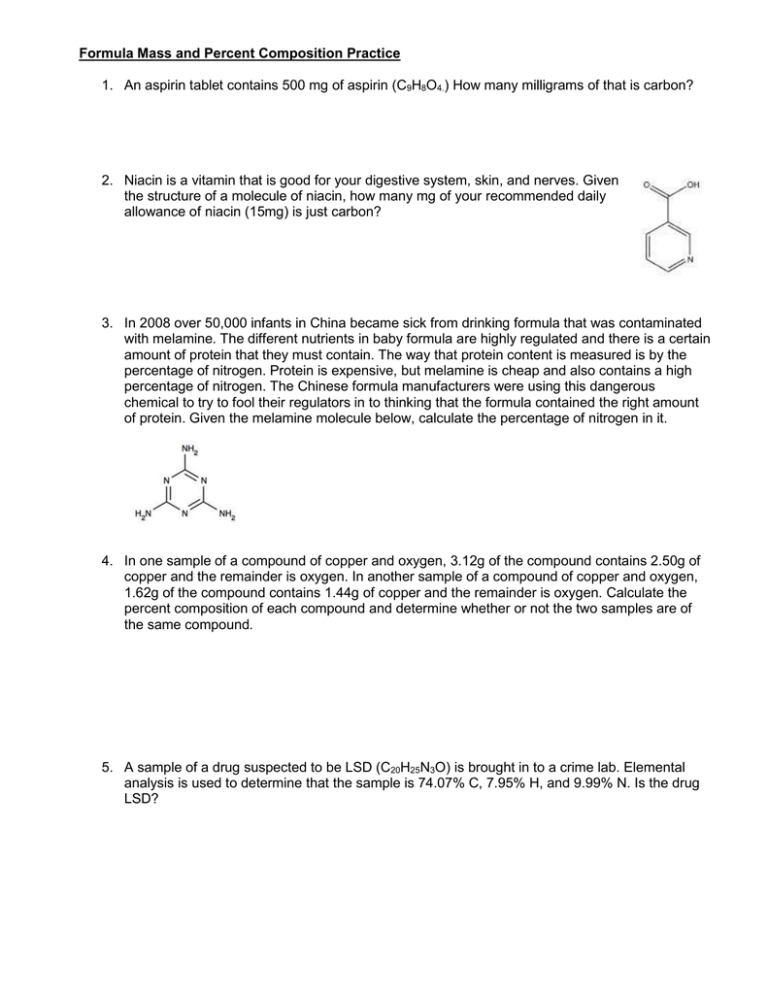
Formula Mass and Percent Composition Practice 1. An aspirin tablet contains 500 mg of aspirin (C9H8O4.) How many milligrams of that is carbon? 2. Niacin is a vitamin that is good for your digestive system, skin, and nerves. Given the structure of a molecule of niacin, how many mg of your recommended daily allowance of niacin (15mg) is just carbon? 3. In 2008 over 50,000 infants in China became sick from drinking formula that was contaminated with melamine. The different nutrients in baby formula are highly regulated and there is a certain amount of protein that they must contain. The way that protein content is measured is by the percentage of nitrogen. Protein is expensive, but melamine is cheap and also contains a high percentage of nitrogen. The Chinese formula manufacturers were using this dangerous chemical to try to fool their regulators in to thinking that the formula contained the right amount of protein. Given the melamine molecule below, calculate the percentage of nitrogen in it. 4. In one sample of a compound of copper and oxygen, 3.12g of the compound contains 2.50g of copper and the remainder is oxygen. In another sample of a compound of copper and oxygen, 1.62g of the compound contains 1.44g of copper and the remainder is oxygen. Calculate the percent composition of each compound and determine whether or not the two samples are of the same compound. 5. A sample of a drug suspected to be LSD (C20H25N3O) is brought in to a crime lab. Elemental analysis is used to determine that the sample is 74.07% C, 7.95% H, and 9.99% N. Is the drug LSD?








![BMF 48: Melamine-[15N3] N N N NH NH H N](http://s2.studylib.net/store/data/018725711_1-017e211c0db09645157cbf7a1c0eb380-300x300.png)

