Molecular geometry
advertisement
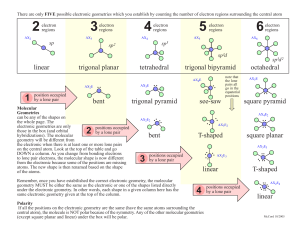
Lab 6 MOLECULAR GEOMETRY Objectives Correlate Lewis dot structures with electron domain geometries View the effect of lone pairs of electrons on molecular shape Materials 5 molecular models representing the 5 basic electron domain geometries 13 balloon models representing the 13 different molecular geometries How to determine the 3D structure of a molecule Step 1: Draw the Lewis structure Step 2: Determine electron-domain geometry around the central atom. Step 3: Determine the molecular geometry Step 2: Determine electron domain geometry VSEPR theory – a model used to predict the 3D structure of individual molecules. This theory is based on the idea that electrons repel each other. Electron domain geometry describes the geometric arrangement of electrons around an atom (bonds and/or lone pair(s) of electrons). Step 2: Determine electron domain geometry There are 5 basic electron domain geometries 1. 2. 3. 4. 5. Linear Trigonal planar Tetrahedral Trigonal bipyramidal Octahedral Step 3: Determine the molecular geometry Linear Trigonal Planar (Bent) Tetrahedral (trigonal pyramid; bent) Trigonal bipyramidal (Seesaw; T shaped) Octahedral (Square pyramid; square planar) (Show some examples and diagrams) Lab 6: Procedure/Data Collection Station 1 – Lewis Structures and Molecular Models - 1 set of eight substances (Table 2 pg 64) are assigned (workshop handout). - In the lab, match the Lewis structure to one of the 5 molecular models on the lab bench. -(Table 3 pg 65) Identify the correct model number (and molecular formula), name the electron-domain geometry, draw the 3D structure, and label all ideal bond angles. Lab 6: Procedure/Data Collection Station 2 – VSEPR and Balloon Models: effect of lone pairs on molecular shape. - Again, you are to match the Lewis structure (from workshop) to one of the balloon models representing VSEPR structures. -Balloons represent the space occupied by either bonding pairs or lone pairs. Station 4 – cont. (Table 4 pg 67 & 68) Identify the VSEPR model number Name the molecular geometry (shape) (e.g. Bent, Seesaw, T shaped etc.) Draw the 3D structure Label all bond angles in your structure, indicating distortions using “<“ or “>” signs. At the end of the lab… Turn in today’s lab write-up and all of the workshop handouts. Lab 9 write-up, naming compounds H.W. and lab 6 (handout from last week) are due in the beginning of the lab.