Scientific Notation & Significant Figures Presentation
advertisement

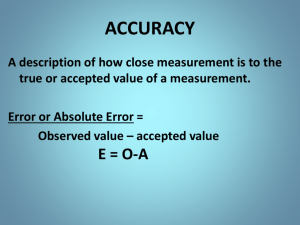
SCIENTIFIC NOTATION Unit 1, Concept 6 Materials Scientific Notation POGIL printouts (3 teams work) Scientific Notation Practice worksheet (1 per student) Key Ideas Rules of Significant figures Rules of 0’s Addition/subtraction Multiplication/division CLO: I can do arithmetic operations and report the results to the correct number of significant figures using a POGIL activity and independent practice Do Now Calculate the density of a silver statue with a mass of 105 g, and a volume of 10 cm3. Do Now Calculate the density of a silver statue with a mass of 105 g, and a volume of 10 cm3. Density = mass/volume = 105 g/10 cm3 = 10.5 g/cm3 Objective I can do arithmetic operations and report the results to the correct number of significant figures using a POGIL activity and independent practice Homework Complete the Significant Figures Practice Worksheet. Due Thursday/Friday Agenda Do Now, Objective (7 min) Accuracy and Precision (10 min) Introduction to Significant Figures (10 min) Team Activity: POGIL (40 min) Significant Figures Review (15 min) Exit Ticket (5 min) Agenda Do Now, Objective (7 min) Accuracy and Precision (10 min) Introduction to Significant Figures Team Activity: POGIL Significant Figures Review Exit Ticket Accuracy vs. Precision Accuracy refers to how close a measured value is to the true value. are you correct? Precision refers to how close a series of measurements are to one another. are your measurements the same every time? Accuracy vs. Precision Precise Instrumentation Less Precise More precise What makes one piece of lab equipment more precise than another? • A piece of lab equipment is more precise than another if it has smaller increments (spaces between numbers) (Ex: A graduated cylinder with 0.5 mL increments is more precise than a graduated cylinder with 1 mL increments) What makes one measurement of more precise than another? • A value with more digits (#’s) is more precise. (3.52 is more precise than 3.5) Accuracy and precision: the target example Accurate and precise Accuracy and precision: the target example Precise, but not accurate Accuracy and precision: the target example Neither accurate, nor precise Accuracy and precision: the target example Accurate, but not precise Agenda Do Now, Objective (7 min) Accuracy and Precision Introduction to Significant Figures (10 min) Team Activity: POGIL Significant Figures Review Exit Ticket Uncertainty and Significant Figures Every measurement is an estimate of the actual value because every measurement contains a degree of uncertainty or error. Error does not mean “mistake”. Error (uncertainty) is the variance between individual measurements that happens when repeated measurements are made on the same sample or object. Uncertainty Example: four students weighed a dime ($0.10) multiple times using different types of balances. The measurements in Set I contain two digits. The first digit (the one’s place) is called the reproducible digit and the second digit in the tenth’s place (0.1) is called the doubtful digit. Agenda Do Now, Objective (7 min) Accuracy and Precision Introduction to Significant Figures Partner Activity: POGIL (40 min) Significant Figures Review Exit Ticket Introduction to POGIL Today we will be doing our first POGIL activity You will be working in groups of 3 to facilitate your own learning around significant figures In a POGIL activity, you will look at data to come to your own understanding of a concept You and your group members will figure out the answers for yourselves, and be able to explain it to Ms. B! POGIL POGIL Group Roles Reader: you will read all information aloud Facilitator: you will lead your group in discussion for each question/task Recorder: you will write down all important information, and answers to questions POGIL: Significant Figures Complete parts B, C, and D of your POGIL activity sheet By the end of this activity you will understand Uncertainty Significant Figures Rounding with Sig Figs Calculations with Sig Figs Part B - Uncertainty An increase in precision DECREASES uncertainty Part C – Significant Figures Non-zero numbers are ALWAYS significant Rules of Zeros Zeros between non-zeros are ALWAYS significant Zeros in the coefficient of scientific notation are ALWAYS significant Zeros after a decimal point are ALWAYS significant Trailing 0’s are not significant Leading 0’s are not significant Part D – Rounding & Calculations with Significant Figures Rules of Significant Figures 1. When multiplying/dividing measurements, the answer must contain the same number of Sig Figs as the measurement with the least Sig Figs 108.4 mi/3.5 gal = 30.97142857 mpg = 31 mpg Rules of Significant Figures 2. When adding/subtracting measurements, the answer must contain the same number of decimal places as the starting measurement with the least amount of decimal places. 7.6 mL + 125 mL = 132.6 mL = 133 mL Agenda Do Now, Objective (7 min) Accuracy and Precision Introduction to Significant Figures Partner Activity: POGIL Significant Figures Review Exit Ticket (5 min) Exit Ticket Complete the Exit Ticket at your desk, and hand it to Ms. Bergman as you leave class. NO calculators! 45 Minute Version of Lesson No POGIL Materials Scientific Notation Practice worksheet (1 per student) Exit Ticket (1 per student) Key Ideas Rules of Significant figures Rules of 0’s Addition/subtraction Multiplication/division CLO: I can do arithmetic operations and report the results to the correct number of significant figures using a notes and independent practice Do Now Calculate the density of a silver statue with a mass of 105 g, and a volume of 10 cm3. Do Now Calculate the density of a silver statue with a mass of 105 g, and a volume of 10 cm3. Density = mass/volume = 105 g/10 cm3 = 10.5 g/cm3 Objective I can do arithmetic operations and report the results to the correct number of significant figures using notes and independent practice Homework Complete the Significant Figures Practice Worksheet. Due Thursday/Friday Agenda Do Now, Objective (7 min) Accuracy and Precision (7 min) Uncertainty (5 min) Significant Figures (10 min) Independent Practice (10 min) Exit Ticket (3 min) Accuracy vs. Precision Accuracy refers to how close a measured value is to the true value Precision refers to how close a series of measurements are to one another. Accuracy vs. Precision Accuracy and precision: the target example Accurate and precise Accuracy and precision: the target example Precise, but not accurate Accuracy and precision: the target example Neither accurate, nor precise Accuracy and precision: the target example Accurate, but not precise Precise Instrumentation • lab equipment is more precise when it has smaller increments (spaces between numbers) (Ex: A graduated cylinder with 1 mL increments is more precise than a graduated cylinder with 10 mL increments) More precise Less Precise Precise Measurements • Numbers with more digits (places) are more precise. • • 3.52 is more precise than 3.5 2.14 is more precise than 2.1 Agenda Do Now, Objective (7 min) Accuracy and Precision (7 min) Uncertainty (5 min) Significant Figures (10 min) Independent Practice (10 min) Exit Ticket (3 min) Uncertainty and Significant Figures Measurements have uncertainty because you never know the EXACT value Uncertainty is the variance between individual measurements that happens when repeated measurements are made on the same sample or object. Uncertainty The pencil is between 25.5 and 26.0 cm long. The uncertainty is ±0.1 cm Agenda Do Now, Objective (7 min) Accuracy and Precision (7 min) Uncertainty (5 min) Significant Figures (10 min) Independent Practice (10 min) Exit Ticket (3 min) Significant Figures Significant figures are those numbers in a measurement that are known with CERTAINTY They matter Significant Figures Four students weighed a dime ($0.10) multiple times using different types of electronic balances. The measurements in Set I contains two digits. • The first digit (the one’s place) is called the reproducible digit • The second digit in the tenth’s place (0.1) is called the doubtful digit. Significant Figures • Reproducible Digit: a number that can be reproduced in multiple measurements. All digits that can be reproduced. • Doubtful Digit: a number that cannot be reproduced in multiple measurements. The last digit in the measurement. Significant Figures Sig Fig Rules 1. 2. 3. Non-zero numbers are ALWAYS significant Rules of Zeros Zeros between non-zeros are ALWAYS significant Zeros in the coefficient of scientific notation are ALWAYS significant Zeros after a decimal point are ALWAYS significant Trailing 0’s are not significant Leading 0’s are not significant Sig Fig Examples Non-zero numbers are ALWAYS significant 234 = 3 sig figs 3 = 1 sig fig 5426 = 4 sig figs Zeros between non-zeros are ALWAYS significant 80.3 = 3 sig figs 3017 = 4 sig figs Zeros in the coefficient of scientific notation are ALWAYS significant 3.008 x 106 = 4 sig figs 7.20 x 102 = 3 sig figs Sig Fig Examples Continued… Zeros after a decimal point are ALWAYS significant Trailing 0’s are not significant 2.0 = 2 sig figs 3.420 = 4 sig figs 700 = 1 sig fig 70180 = 4 sig figs Leading 0’s are not significant 0.0034 = 2 sig figs 0.09 = 1 sig fig Multiplying & Dividing Sig Figs 1. When multiplying/dividing measurements, the answer must contain the same number of Sig Figs as the measurement with the least Sig Figs 108.4 mi/3.5 gal = 30.97142857 mpg = 31 mpg Adding & Subtracting with Sig Figs 2. When adding/subtracting measurements, the answer must contain the same number of decimal places as the starting measurement with the least amount of decimal places. 7.6 mL + 125 mL = 132.6 mL = 133 mL Sig Fig Independent Practice Get started on your Sig Fig Practice Worksheet 1. – a, c, e, g, i, k, m, o 2. – a, c, e 3. – a, c 4. – a, c, e, g 5. a, c, e 6. entire problem Exit Ticket Complete the Exit Ticket at your desk, and hand it to Ms. Bergman as you leave class. NO calculators!