(C) the lack of hydrogen bonding in 1-butanol
advertisement

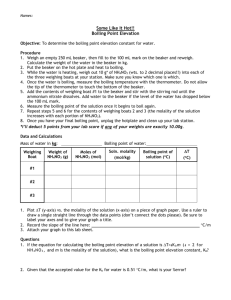
Colligative Properties and IMF Review 1. Which of the following lists these compounds from lowest to highest vapor pressure? a. CH4 < C2H5OH < C2H5-O-C2H5 < Ne b. Ne < CH4 < C2H5-O-C2H5 < C2H5OH c. C2H5OH < C2H5-O-C2H5 < Ne < CH4 d. C2H5-O-C2H5 < C2H5OH < CH4 < Ne e. C2H5-O-C2H5 < C2H5OH < Ne < CH4 Ethanol has H-bonding IMF’s, ethers are polar so have D-D IMF’s, Ne has LDF but has more electrons than methane which also has LDF. 2. The molal boiling point constant for ethyl alcohol is 1.22 oC/molal. Its boiling point is 78.4oC. A solution of 14.2 g of a nonvolatile nonelectrolyte in 264 g of the alcohol boils at 79.8 oC. What is the molar mass of the solute? 46.7 g/mol 3. As you go down the noble gas family on the periodic table, the boiling point increases. This trend is due mainly to a. An increase in hydrogen bonding. b. A decrease in dipole-dipole forces. c. The lower atomic masses as you go down the family. d. An increase in London Dispersion Forces e. An increase in ion-dipole forces. More electrons means more polarizability of those electrons and higher LDF’s 4. What is the expected change in the freezing point of water in a solution of 62.5 g of barium nitrate, Ba(NO3)2, in 1.00 kg of water? (Kf = -1.86 C/m for water) – 1.3 oC 5. A solution is made by dissolving 25 g of NaCl in enough water to make 1.0L of solution. Assume the density of the solution is 1.0 g/cm3. Calculate the mole fraction of the solution. 0.0078 6. The freezing point of an aqueous sodium chloride solution is –20.0oC. What is the molality of the solution? (Kf = -1.86 C/m for water) 5.37 m 7. Explain the following in terms of the bonding of the compounds involved: At ordinary conditions, HF (normal boiling point = 20°C) is a liquid, whereas HCl (normal boiling point = 114°C) is a gas. Remember, you need to list both types of IMFs, use the word between appropriately, and draw a conclusion based on the evidence. Two HF molecules contain hydrogen bonding between the molecules, whereas HCl molecules interact through dipole dipole interactions. Hydrogen bonding is a significantly stronger intermolecular force than the dipoledipole interactions and so the boiling point of HF is higher than HCl. 8. The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions. (a) What does point V represent? What characteristics are specific to the system only at point V? Triple point. Solid, liquid, and gas are present in a dynamic equilibrium. (b) What does each point on the curve between V and W represent? This is the liquid-vapor line so each point is the boiling point at a specific pressure. (c) Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere. The substance begins as a solid. At point Y the solid sublimes and the solid and gas are in dynamic equilibrium. At point Z the substance is a gas. (d) In a solid-liquid mixture of this substance, will the solid float or sink? Explain. The solid will sink since at higher pressures (higher density) the substance turns into a solid. This means that the solid is more dense than the liquid. 9. Which of the following describes the changes in forces of attraction that occur as H2O changes phase from a liquid to a vapor? (A) (B) (C) (D) H-O bonds break as H-H and O-O bonds form. Hydrogen bonds between H2O molecules are broken. Covalent bonds between H2O molecules are broken. Ionic bonds between H+ ions and OH- ions are broken. (E) Covalent bonds between H+ ions and H2O molecules become more effective. 10. The approximate boiling points for hydrogen compounds of some elements in the nitrogen family are: (SbH3 15ºC), (AsH3 –62ºC), (PH3–87ºC), and (NH3 –33ºC). The best explanation for the fact that NH3 does not follow the trend of the other hydrogen compounds is (A) NH3 is the only one to exhibit hydrogen bonding (B) NH3 is the only one that is water-soluble (C) NH3 is the only one that is nearly ideal in the gas phase (D) NH3 is the only one that is a base (E) NH3 is the only one that is nonpolar 11. Which of the following best explains why 1-butanol, CH3CH2CH2CH2OH, has a higher surface tension than its isomer, diethyl ether, CH3CH2OCH2CH3? (A) the higher density of 1-butanol (B) the lower specific heat of 1-butanol (C) the lack of hydrogen bonding in 1-butanol (D) the higher molecular mass of 1-butanol (E) the presence of hydrogen bonding in 1-butanol 12. Pick the answer that most likely represents the substances’ relative solubilities in water. (A) CH3CH2CH2CH3 CH3CH2CH2OH HOCH2CH2OH (B) CH3CH2CH2OH CH3CH2CH2CH3 HOCH2CH2OH (C) CH3CH2CH2CH3 HOCH2CH2OH CH3CH2CH2OH (D) HOCH2CH2OH CH3CH2CH2OH CH3CH2CH2CH3 (E) CH3CH2CH2OH HOCH2CH2OH CH3CH2CH2CH3 13. What is the energy change that accompanies the conversion of molecules in the gas phase to a liquid? (A) heat of condensation (B) heat of deposition (C) heat of sublimation (D) heat of fusion (E) heat of vaporization 2005 B Answer the following questions about a pure compound that contains only carbon, hydrogen, and oxygen. (a) A 0.7549 g sample of the compound burns in O2(g) to produce 1.9061 g of CO2(g) and 0.3370 g of H2O(g). (i) Calculate the individual masses of C, H, and O in the 0.7549 g sample. (ii) Determine the empirical formula for the compound. (b) A 0.5246 g sample of the compound was dissolved in 10.0012 g of lauric acid, and it was determined that the freezing point of the lauric acid was lowered by 1.68˚C. The value of Kf of lauric acid is 3.90˚C m–1. Assume that the compound does not dissociate in lauric acid. (i) Calculate the molality of the compound dissolved in the lauric acid. (ii) Calculate the molar mass of the compound from the information provided. (c) Without doing any calculations, explain how to determine the molecular formula of the compound based on the answers to parts (a)(ii) and (b)(ii). Answer: 12 (a) (i) 1.9061 g 44 2 0.3370 g H2O 18 = 0.5198 g of C = 0.03744 g of H 0.7549 g sample –0.5198 g C –0.0374 g H 0.1977 g of Oxygen 1000 mmol (ii) 0.5198 g C 12.0 g = 43.32 mmol C 1000 mmol 0.0374 g H = 37.4 mmol H 1.0 g 1000 mmol 0.1977 g O 16.0 g = 12.36 mmol O dividing each by 12.36 gives 3.5 C : 3 H : 1 Oxygen, OR 7 C : 6 H : 2 O, thus an empirical formula of C7H6O2 ∆Tf 1.68˚C (b) (i) molality = K = = 0.431 m 3.90˚C m–1 f g of solute 0.5246 g (ii) molar mass = kg solvent•molality = 0.0100012 kg • 0.431 m = 122 g/mol (c) from (a)(ii), the molar mass of C7H6O2 = 122.0 g/mol since the two molar mass are the same, the empirical formula = molecular formula 2001 D Required Answer the questions below that relate to the five aqueous solutions at 25C shown above. (a) Which solution has the highest boiling point? Explain. (b) Which solution has the highest pH? Explain. (c) Identify a pair of the solutions that would produce a precipitate when mixed together. Write the formula of the precipitate. (d) Which solution could be used to oxidize the Cl–(aq) ion? Identify the product of the oxidation. (e) Which solution would be the least effective conductor of electricity? Explain. Answer: (a) solution 1, Pb(NO3)2. This compound will dissociate into three ions with the highest total particle molality. The greater the molality, the higher the boiling point. Solutions 2, 3, and 5 will produce two ions while solution 4 is molecular. (b) solution 5, KC2H3O2. The salt of a weak acid (in this case, acetic acid) produces a basic solution, and, a higher pH. (c) solution 1, Pb(NO3)2, and solution 2, NaCl. PbCl2 (d) solution 3, KMnO4 , ClO3– (e) solution 4, C2H5OH. Ethyl alcohol is covalently bonded and does not form ions in water. Therefore, the solution is not a better conductor of electricity than water, which is also covalently bonded. 1. Explain each of the following in terms of atomic and molecular structures and/or intermolecular forces. (c) The normal boiling point of CCl4 is 77 °C, whereas that of CBr4 is 190 °C. Both compounds have a tetrahedral geometry so and are non-polar. The only intermolecular force present in both compounds is London dispersion forces. (You might be tempted to say that CBr4 has a higher molecular weight here but don’t.) CBr4 contains more polarizable (remember this means squishy) electrons and therefore it has a strong LDF between two CBr4 molecules that are present between two CCl4 molecules, therefore, CBr4 has a higher boiling point. (d) NaI(s) is very soluble in water whereas I2(s) has a solubility of only 0.03 gram per 100 grams of water. Sodium iodide forms strong ion dipole interactions with water. These interactions must release more energy than it takes to break the lattice of NaI. Iodine is a non-polar molecule that can only interact with water through London Dispersion Forces. The London Dispersion Forces create must not release as much energy as it takes to break the LDF’s between the iodine molecules. (Notice, we did not say “Like dissolves like”. It rarely gets credit.) I suspect that you would get a point for identifying the correct IMF present and another one for discussing energy is required to break a bond and it is returned when the bond is made.) 4) Discuss the following phenomena in terms of the chemical and physical properties of the substances involved and general principles of chemical and physical change. (a) As the system shown below approaches equilibrium, what change occurs to the volume of water in beaker A ? The volume of water in beaker A would decrease because the vapor pressure of A would be higher due it not having a solute (Raoult’s Law). The vapor more easily escapes the solution than it does in beaker B, so the volume level in beaker A would decrease. What happens to the concentration of the sugar solution in beaker B ? Explain why these changes occur. The concentration in beaker B would decrease. The increase in water vapor in the bell chamber from evaporation in A would condense into beaker B causing the concentration to decrease. (b) A bell jar connected to a vacuum pump is shown on the right. As the air pressure under the bell jar decreases, what behavior of water in the beaker will be observed? Explain why this occurs. More water molecules will evaporate into the air above the chamber. The pressure from air above the beaker is decreasing allowing more water vapor to fill that space. (c) A water solution of I2 is shaken with an equal volume of a nonpolar solvent such as TTE (trichlorotrifluoroethane). Describe the appearance of this system after shaking. (A diagram may be helpful.) Account for this observation. The iodine, being non-polar, will go into the non-polar TTE layer to escape the polar water. TTE would likely have a brownish purple coloring due to the iodine. (Another like dissolves like question.) So how do you know that TTE is non-polar. It is not so easy. A drawing of its Lewis structure would hint that it would have a little bit of polarity. I guess a general understanding that water is one of the most polar solvents available is something that is good to know.