Electron Configuration
advertisement

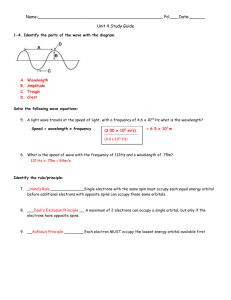
Ch 11 - Electron Configuration Radiant Energy Waves • Light travels as both • Waves and Packets of energy. • These packets are called photons. Light is a form of Electromagnetic Radiation. – EM Radiation has waves in the electric and magnetic fields Electromagnetic waves • Electromagnetic waves have two basic parts. 1. electric field 2. magnetic field • The fields are perpendicular to each other. Waves • All waves (Water or Electromagnetic) have 4 key characteristics: – Amplitude – Wavelength – Period – Frequency Wave Characteristics • Amplitude. – Height of a wave from origin to a peak/crest. – Affects brightness and intensity. • Wavelength. – Distance from crest to crest. Distance for one full cycle. – Visible light: 400-750nm. Wave Characteristics • Period – time that it takes to complete a full cycle. – Measured in seconds • Frequency. – number of cycles per second. – Measured in hertz(Hz) – High frequency = high energy Wave Characteristics • Speed of light – Speed of light a constant: 3.00 X 108 m/s. • Frequency and Wavelength related by the equation: =c/ Wavelength and frequency You can also find the frequency by rearranging the equation: = c / • First, multiply by (frequency): = (c/) • Now, divide by (wavelength): () / = c / • Leaving: = c/ Moving on…. Try this… Remember: = c / 1) If the frequency of a wave is 93.1 x 106 , what is the wavelength? 2) If the wavelength of a wave is 1.54 m, what is it’s frequency? Try this… 1. Split up into groups of 3. 2. Draw one wave with wavelength 4 cm 3. Draw second wave with wavelength twice of the first wave. 4. Draw a third wave with 3 times the wavelength the first wave. 5. Draw 3 more waves with the same wavelengths as the first set but with an amplitude of 6 cm. Try this… 1. Order the waves from lowest to highest frequency. 2. Order the waves from lowest to highest energy. 3. Order the waves from lowest to highest amplitude. Electromagnetic Spectrum • Many parts including: – – – – – – – Gamma Rays (10-11 m) X-Rays (10-9 m) Ultra-violet (10-8 m) Visible (10-7 m) Infared (10-6 m) Microwave (10-2 m) TV/Radio (10-1 m) Electromagnetic Spectrum • Visible Spectrum: ROY G BIV – Red – Orange – Yellow – Green – Blue – Indigo – Violet Electromagnetic Spectrum (once more) Wavelength practice… Remember: = c / For the following questions assume that the speed of light is = 3000m/s 1) If the frequency of a wave is 847 Hz, what is the wavelength? 2) If the frequency of a wave is 4,985 Hz, what is the wavelength? 3) If the frequency of a wave is 290 Hz, what is the wavelength? 4) If the frequency of a wave is 38,759 Hz, what is the wavelength? Wavelength practice… Remember: = c / For the following questions assume that the speed of light is = 3000m/s 1) If the wavelength of a wave is 1.54 m, what is it’s frequency? 2) If the wavelength of a wave is .875 m, what is it’s frequency? 3) If the wavelength of a wave is 3.39 m, what is it’s frequency? 4) If the wavelength of a wave is .657 m, what is it’s frequency? Electron Configuration Quantum Theory Early Puzzlements • Wave model for light was originally accepted by scientific community. • This couldn’t explain why metals heating first emitted invisible radiation and then visible radiation. • Other questions included why elements only emitted certain characteristic colors of light. Terminology • Ground State – when an atom is at the lowest possible energy state. • Excited State – when an atom has excess energy Line Spectra • Def: A spectrum that contains only certain colors/wavelengths. • AKA: The Atomic Emission Spectrum • Each element has it’s own “fingerprint” emission spectrum. Line Spectra • Assume you “energize” some H atoms. • There are only certain types of photons emitted. • We see only selected colors that correspond to these photons energy levels. Line Spectra • Each photon has a frequency that is proportional to the change in energy of the electron. Plank’s Theory • Every object can only absorb or emit a fundamental amount of energy. • This amount is called a quantum. • The amount is like moving up or down steps. Plank’s Theory • Plank’s Theory is based on the relationship between frequency and the energy of the particle. • Energy = h x frequency • E = h • Plank’s Constant: – h = 6.6262 X 10-34 J-s Dual Nature of Radiant Energy • Proven in 1923 by Arthur Compton – Showed photon could collide with an electron like tiny balls. • Summary: – Light behaves as a wave ( = c/) – Light behaves as a particle (E = h) Electron Configuration Another Look at the Atom The Bohr Model • Bohr drew the connection between Rutherford's model of the atom and Planks idea of quantization. • Energy levels labeled with Quantum Numbers (n) • Ground state, or lowest energy level – n=1 • Excited State – level of higher energy Matter Waves • If energy has dual nature, why not matter? • De Broglie thought so. – Matter Waves – the wavelike behavior of waves. – Didn’t stand without experimental proof • Davison and Germer proved this with experiments in 1927. • Why don’t we see these matter waves? Mass must be very small to observe wavelength. Heisenberg Uncertainty • Uncertainty Principle – The position and momentum of a moving object cannot simultaneously be measured and known exactly. • Translation: – Cannot know exactly where and how fast an electron is moving at the same time. Electron Configurations A New Approach to the Atom Quantum-mechanics Model • Includes all the ideas of the atom we have covered: – Energy of electrons is quantized – Electrons exhibit wavelike behavior – Electrons position and momentum cannot be simultaneously known – Model does describe the probable location of electrons around the nucleus Probability and Orbitals • Electron Density: – The density of an electron cloud. • Atomic Orbitals: – A region around the nucleus of an atom where an electron with a given energy is likely to be found. • Kinds of orbitals: – Each kind has own different basic shape. – Given letter designations of s, p, d and f. – s-orbitals are spherical – p-orbitals are dumbbell – d- and f-orbitals more complex. Probability and Orbitals • Electron Density: – The density of an electron cloud. • Atomic Orbitals: – A region around the nucleus of an atom where an electron with a given energy is likely to be found. – Orbitals are nothing like orbits of a planet! Probability and Orbitals • Lets look at the cool animation… Orbitlas and Energy • Principle energy levels (n) can be divided into sublevels. • Number of sublevels is equal to the number of the principle energy level. Orbitals and Energy • Each sublevel has one or more orbitals – – – – s – one P – three d – five f – seven • Summary provided on pg 372-374 Electron Spin • Electrons have two spins: – Up or clockwise – Down or counterclockwise • Only two electrons (one of each spin) can occupy an orbital. These electrons are said to be “paired”. Electron Configurations Electron Configurations Electron Configurations • Notation which shows how the electrons are distributed among the various atomic orbital and energy levels. 1s2 How it works 2 1s • “1” refers to the principle quantum number “n=1” • This “n=1” stands for the energy level • The electrons occupy the first energy level of the atom How it works 2 1s • “s” refers to the angular momentum. • Tells us electrons occupy an “s” or spherical orbital How it works 2 1s • “2” refers to the total number of electrons in that orbital (s) How it works 2 1s • Summary • There are two electrons(2) in the spherical orbital (s) at the fist energy level (1) Reminder/Review • Principle energy levels (n) can be divided into sublevels. • Number of sublevels is equal to the number of the principle energy level. Orbitals • An orbital is a space that can be occupied by up to two electrons. • Each sublevel holds different number of orbitals. Sublevel # of orbitals Maximum # s p d f 1 3 5 7 of electrons 2 6 10 14 Orbitals • To calculate # of orbitals: – # of orbitals = n2 • ex. Thrid energy level (principle level) (n=3) – # of orbitals = 32 – # of orbitals = 9 – 3 sublevels (s+p+d) => (1+3+5), see above chart Orbitals • To calculate # of electrons: – # of electrons = 2n2 • ex. fourth energy level (principle level) (n=4) – # of orbitals = 2(4)2 – # of orbitals = 32 Filling Sublevels with Electrons • There is a specific order that energy sublevels fill up. Follow the chart. • Or…. • Read the PT like a book! Filling Sublevels with Electrons • Read the PT like a book! • Remember: – d elements move up 1 principle # – f elements move up 2 principle # Practice Let’s practice!!! • Oxygen • Vandium • Europium Example • Oxygen – 1s2 2s2 2p4 • Vandium – 1s2 2s2 2p6 3s2 3p6 4s2 3d3 • Europium – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f6 Short-hand Notaion • • • • • Very similar to electron configuration Start at the selected element Move backward till you are at a noble gas. Write the noble gas in brackets Continue as normal. Example • Oxygen – 1s2 2s2 2p4 • Vandium – 1s2 2s2 2p6 3s2 3p6 4s2 3d3 – [Ar] 4s2 3d3 • Europium – 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f6 – [Rn] 6s2 4f6 Let’s practice!!! • Oxygen • Vandium • Europium Try these on your own!!! Write the long-hand and short-hand electron notation for the following elements. 1. 2. 3. 4. 5. Beryllium Fluorine Silicon Manganese Gallium 6. Silver 7. Samarium 8. Gold 9. Bismuth 10.Uranium Orbital Notaion • • • • • Very similar to electron configuration Just use a box instead of a superscript Boxes represent orbitals Arrows represent electrons Put electrons in “up” first, then “down”. DON’T FORGET!!! • An orbital is a space that can be occupied by up to two electrons. • Each sublevel holds different number of orbitals. Sublevel # of orbitals Maximum # s p d f 1 3 5 7 of electrons 2 6 10 14 Orbital Notaion • Remember only two electrons per box • Ex. F • Ex. P Practice • • • • Li C Al S Lewis Dot Diagram • consists of an elemental symbol and… • the valence electrons – outer-most electrons of the atom – valence electrons are the available electrons that can be involved in bonding. Lewis Dot Diagram • If you don’t know how many electrons are in the valence shell then, write the electron configuration! • Look at the last principal number. • Li: 1s2 2s1 • S: 1s2 2s2 2p6 3s2 3p4 • Kr: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 Lewis Dot Diagram • How to make them – there are multiple ways – we are going to use a specific order • Ex. O Practice • • • • Li C Al S Electron Notation Poster • Once you have your group come to me to get your Element. Extra practice!!!! If an atom has 16 protons and 30 neutrons and a charge of -2 • What is the atomic number?_____ • What is the atomic mass?_______ • What is the mass number?______ • How many electrons does it have?_____ • What is the element symbol?______ • What kind of ion is it?________ If an atom has 19 protons and 42 neutrons and a charge of +1 • What is the atomic number?________ • What is the atomic mass?_______ • What is the mass number?_________ • How many electrons does it have?______ • What is the element symbol?______ • What kind of ion is it?_______ Write two symbols on the board. • • • • • • • How many protons are there?______ How many neutrons are there?______ How many electrons are there?_____ What is the atomic number?______ What is the atomic mass?______ What is the mass number?_______ What kind of ion is it? ________ Label the Periodic Table!!! Make scientist map!!! Remember: = c / E = h =c/ 1)If the wavelength of a wave is 3.39 m, what is it’s frequency?________ 2)If the wavelength of a wave is .657 m, what is it’s frequency?________ 3)If the frequency of a wave is 4,985 Hz, what is the wavelength?_______ 4)If the frequency of a wave is 290 Hz, what is the wavelength?_______ QUIZ • Clear your desk of everything except a pen/pencil and paper 1)Write the abbriviated electron notation of Ru (atomic # 44) 2)Write the unabbriviated electron notation of O (atomic # 8) 3)Write the orbital notation of C (atomic # 6) 4)Write the Lewis Dot Diagram of N (atomic # 7)