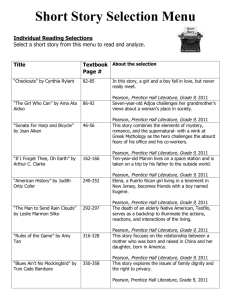
Chapter 11 Chemical Reactions
Activities and resources
•Writing Chemical equations
Lab –chemical reactions
•Balancing Equations
Workshops
•Classifying reactions
TB & Workbooks
•Predicting the products
Animations and games
for balancing equations
and types of reactions:
•Reactions in aqueous
solutions (complete and net
ionic equations)
•Predicting the formation of a
precipitate
http://funbasedlearning.com/che
mistry/chemBalancer/ques5.htm
http://www.youtube.com/watch?v=iHHvx1VC_8&NR=1
http://www.youtube.com/watch?v=tE
4668aarck&feature=related
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
Word Equations
To write a word equation, write the
names of the reactants to the left of the
arrow separated by plus signs; write the
names of the products to the right of the
arrow, also separated by plus signs.
Reactant + Reactant Product + Product
Slide
2 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
Methane + Oxygen Carbon dioxide + Water
(the reaction between methane and oxygen can
be described as a combustion reaction.)
Slide
3 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
iron + oxygen iron(III) oxide
(the reaction between Fe and O2 to form Fe2O3
can be described as a combination or synthesis
reaction)
Slide
4 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
Chemical Equations
A chemical equation is a representation of a
chemical reaction; the formulas of the reactants
(on the left) are connected by an arrow with the
formulas of the products (on the right).
Slide
5 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
Slide
6 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
A skeleton equation is a chemical equation
that does not indicate the relative amounts
of the reactants and products. (i.e. the
equation is not yet balanced)
Here is the equation for rusting:
Fe + O2 Fe2O3
How would you balance this equation?
Slide
7 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Writing Chemical Equations
A catalyst is a substance that speeds up the
reaction but is not used up in the reaction.
Without Catalyst
With Catalyst
Slide
8 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
9 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
10 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
11 of 37
© Copyright Pearson Prentice Hall
End Show
Practice Problems for Conceptual Problem 11.1
Slide
12 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Balancing Chemical Equations
Balancing Chemical Equations
What are the steps in writing a balanced
chemical equation?
To write a balanced chemical equation,
first write the skeleton equation. Then
use coefficients to balance the equation
so that it obeys the law of conservation
Slide
of mass.
13 of 37
© Copyright Pearson Prentice Hall
End Show
11.1
Describing Chemical
Reactions
>
Balancing Chemical Equations
A chemical reaction is also described by a
balanced equation in which each side of the
equation has the same number of atoms of each
element and mass is conserved.
Slide
14 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
15 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
16 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
17 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
18 of 37
© Copyright Pearson Prentice Hall
End Show
Go to following website for balancing
chemical equation game:
http://funbasedlearning.com/chemistry/chemBalancer/ques5.htm
Complete worksheet (class handout) and
turn in for grading
Slide
19 of 37
© Copyright Pearson Prentice Hall
End Show
Practice Problems for Conceptual Problem 11.2
Slide
20 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
21 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
22 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
23 of 37
© Copyright Pearson Prentice Hall
End Show
Slide
24 of 37
© Copyright Pearson Prentice Hall
End Show
Practice Problems for Conceptual Problem 11.2
Slide
25 of 37
© Copyright Pearson Prentice Hall
End Show
11.1 Section Quiz.
Assess students’ understanding
of the concepts in Section 11.1.
Continue to:
-or-
Launch:
Section Quiz
Slide
26 of 37
© Copyright Pearson Prentice Hall
End Show
11.1 Section Quiz.
1. Propane gas reacts with oxygen to produce
water vapor and carbon dioxide. Choose the
correct word equation for this reaction.
a. propane + carbon dioxide water +
oxygen
b. propane + oxygen + water carbon
dioxide
c. propane + oxygen + water + carbon
dioxide
d. propane + oxygen water + carbon
dioxide
Slide
27 of 37
© Copyright Pearson Prentice Hall
End Show
11.1 Section Quiz.
2. Which of the following is a skeleton equation?
a. H2 + CO CH3OH
b. 2H2 + CO CH3OH
c. 2H2 + CO2 CH3OH
d. hydrogen + carbon monoxide methanol
Slide
28 of 37
© Copyright Pearson Prentice Hall
End Show
11.1 Section Quiz.
3. What coefficient for H2SO4 is required to
balance the following equation?
Ca3(PO4)2 + ____ H2SO4 3CaSO4 + 2H3PO4
a. 1
b. 2
c. 3
d. 4
Slide
29 of 37
© Copyright Pearson Prentice Hall
End Show