Solutions (4.1)
advertisement
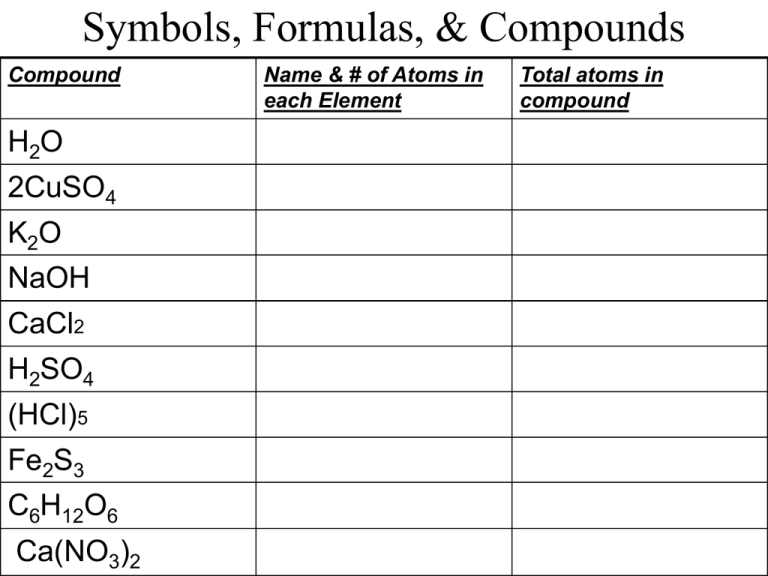
Symbols, Formulas, & Compounds Compound H2O 2CuSO4 K2O NaOH CaCl2 H2SO4 (HCl)5 Fe2S3 C6H12O6 Ca(NO3)2 Name & # of Atoms in each Element Total atoms in compound Mixing it Up NCSCOS 4.05 Chapter 4-1D ~ Messana Compounds & Mixtures Today’s vocab terms: Mixture, solution, solute, Solvent, suspension, Freezing point, boiling point Solutions ARE Mixtures MIXTURE: combinations of substances, like a salad. Easily separated into basic components. SOLUTION: type of mixture that can not be separated. Solutions are blended together so well that they become one. Known as a homogenous mixture. Parts of a Solution Solutions have two definite components: 1. SOLUTES: the substance that is dissolved to make a solution; the solute dissolves and is usually the smaller component 2. SOLVENT: the substance that dissolves the solute; usually the larger component Ex: Kool- Aid Solute (sugar) + Solvent (water) = SOLUTION (Kool- Aid) Types of Solutions 3 types of solutions: 1. Liquid Solution: water contains many dissolved substances 2. Solid solution: bronze is a metal solution consisting of tin dissolved in copper 3. Gas Solution: air around you is a solution Suspensions In a SUSPENSION, the particles are larger than those found in the solution; instead of dissolving ,the particles turn the mixture cloudy. Ex: Flour in water Suspensions can be easily separated. How could this solution be separated? Changing Properties Adding a solute will: 1. Lower the FREEZING POINT (the temperature at which a liquid turns into a solid)Ex: SALT added will make it more difficult to freeze 2. Raises the BOILING POINT (the temperature at which a liquid turns into a gas) Ex: SALT added to water will make it take longer to boil…but water will be hotter! What are examples of solutions? 1.oxygen gas is dissolved in seawater 2. air is oxygen dissolved in nitrogen 3. bronze consists of tin dissolved in copper (solid solution) 4. carbon dioxide gas dissolved in soda 5. vinegar is acetic acid in water (liquid solution) POP QUIZ!!!! Please put on back of Notesheet 1. 2. 3. 4. What are the two parts of a solution? Which part of a solution gets dissolved? Which part of a solution dissolves the other? Which type of bonding occurs when electrons are shared? POP QUIZ 5. Which type of solution has charged particles that interact with the solvent? 6. What happens to the freezing point of a compound when you add a solute? 7. What happens to the boiling point when you add a solute? 8. What is a subscript, where is it located, and what does it tell you? 9. What are the three types of solutions? BONUS: What is known as the temperature at which a solid becomes a liquid?