Solutions
advertisement

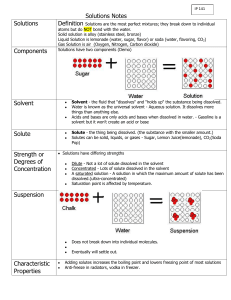
Solutions A solution is a mixture of one substance dissolved in another so the properties are the same throughout. Particles dissolve into the mixture (they get very small and spread out). Solutions are homogeneous. The particles do not settle and will pass right through a filter (like a coffee filter). A solution is composed of a solute and the solvent. The solute is the substance being dissolved. The solvent is the part of the solution that does the dissolving. Water is often called the universal solvent because it dissolves so many substances. It dissolves sugar, salt, oxygen, and carbon dioxide. Examples of Solutions: Gas Gas Oxygen and other gases in nitrogen (air) Carbon dioxide in water Liquid (carbonated water) Solid Hydrogen dissolved to palladium Liquid Water vapor in air (humidity) Solid The odor of a solid -molecules of that solid being dissolved in the air Ethanol (common alcohol) in water. Sucrose (table sugar) in water; sodium chloride (table salt) in water Water in activated charcoal Steel, Brass, other metal alloys Making Solutions So, what happens? How do you make that solution? Mix the two liquids and stir. It's that simple. Science breaks it into three steps. When you read the steps, remember... Solute=Sugar (Yellow Dots) Solvent=Water (Blue) System=Glass. 1. The solute is placed in the solvent and the solute slowly breaks into pieces. If you start to stir the liquid, the mixing process happens much faster. 2. The molecules of the solvent begin to move out of the way and they make room for the molecules of the solute. Example: The water has to make room for the sugar molecules to spread out. 3. The solute and solvent interact with each other until the concentration of the two substances is equal throughout the system. The concentration of sugar in the water would be the same from a sample at the top, bottom, or middle of the glass.