Ch. 14/15 Checklist

Study Checklist for Ch. 14 & 15 Test (from the chapter highlight pages :-)
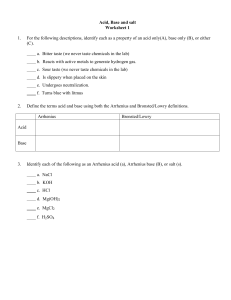
Know your vocabulary – Pg. 490 & Pg. 522
Section 14.1
Properties of Acids/Bases
Do you know how to recognize an Arrhenius Acid or Base?
How is the strength of an Arrhenius acid/based determined (Pg. 490)
Section 14.2
What is a Bronsted-Lowry acid and base?
What is the difference between a monoprotic and polyprotic acid?
Section 14.3
Review conjugate acid-base pairs (notes & practice worksheet)
How does having a strong acid relate to the strength of its conjugate base?
Why would you write a double arrow for the ionization of an acid?
Know what a neutralization reaction produces
Pg. 501 List of strong acids/bases you should know
How does adding more O’s on a oxyacid group affect its strength?
What is an amphoteric compound?
Acid Rain
Section 15.1
know how to calculate pH , pOH, hydronium and hydroxide concentrations
**must show all work on test**
How does having an acid or base that donates more than 1 H+ or OH- affect the concentration of the ions, if given Molarity?
pH scale
Section 15.2
don’t need to memorize acid/base indicators, but know what they are used for
be able to interpret a titration curve: know how to identify starting solution, added standard, indicator to use, pH of equivalence pt., amount of added standard at equivalence pt.
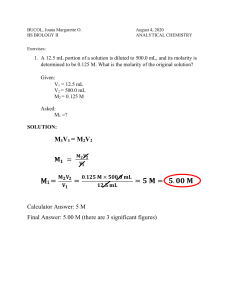
Know how to determine the molarity or volume of a solution using M
1
V
1
=
M
2
V
2
My study strategy…
Checkout the PowerPoint online and use it to guide you thorough your notes and practice worksheets, making sure you understand each of the bullet points mentioned above.