States of Matter
advertisement

States of Matter
States of matter
• Gases, liquids, and solids are the three states of matter.
• Certain asymmetric molecules (surfactants) commonly
exhibit a fourth phase called the mesophase which is
between the liquid and solid state. Therefore the fourth
state of matter is the Liquid Crystalline state.
Gas State
• Gases are described as molecules that have kinetic
energy that produces rapid motion.
• Gas molecules exert relatively small forces on each other
(molecules try to act independently of one another).
• A gas mixes completely with any other gas.
• They move in random and vigorous motion bouncing of
the walls of the container. This constant motion produces
a pressure called vapor pressure. It is measured in atm,
mm (cm) of mercury or dyne/cm2.
Gas State
• Gas is the only state that is compressible.
• A gas uniformly fills any container and assumes its shape
(volume).
• It is important to understand gas laws in the development
of pharmaceutical aerosols, anesthetics, and inhalers.
The Ideal Gas Law
PV=nRT
• The ideal gas law shows the relation between the
volume, pressure, temperature, and the number of moles
of the gas.
• R is the gas law constant (1.98717 cal.K-1.mol-1, 8.3143 J
.K-1.mol-1, or 0.082 L.atm.K-1mol-1, to be selected based
on the other units in the equation).
• The ideal gas law is an equation of state for a gas, where
the state of the gas is its condition at any given time. A
particular state of a gas is described by its pressure,
volume, temperature, and number of moles. Knowledge of
any three of these properties is enough to completely
define the state of a gas, since the fourth property can
then be determined from the equation for the ideal gas.
The Ideal Gas Law
PV=nRT
• To obtain the numeric value of R, the following procedure
is followed. If 1 mole of an ideal gas is chosen, its volume
under standard temperature and pressure (STP) (i.e. at
0ºC (273.15 K) and 760mmHg (1 atm) has been found to
be 22.4 liters. When P is 1 atm, V=22.4 L (volume of 1
mole of an ideal gas), and T is 273.15 K, the gas constant
is 0.082 L.atm.K-1mol-1.
• See example 2-2 page 25 and solve problem 2-3 page 50
– fourth edition of Martin. If you have the fifth edition, see
example 2-2 page 28 and solve problem 2-3 page 685. If
you have the sixth edition see example 2-2 page 23.
The Ideal Gas Law
PV=nRT
Example:
One mole of water is converted to steam at 100 oC
at 1 atm. What is the volume of the steam?
T(K) = T(C)+273 = 100+273 =373
V = n RT/P
= 1 mol x 0.082 LatmK-1mol-1373 K / 1 atm = 30.6
L
The Ideal Gas Law
PV=nRT
• The ideal gas law can be very useful when one needs to
find the approximate molecular weight of a gas. The n is
substituted for g/M, which is grams of the gas/ molecular
weight.
• See example 2-3 page 26 in the fourth edition (if you
have the fifth edition see example 2-3 page 28. If you
have the sixth edition see example 2-3 page 24).
• Solve problem 2-4 page 50 in the fourth edition (if you
have the fifth edition solve problem 2-4 page 685. If you
have the sixth edition solve problem 2-3).
Kinetic Molecular Theory
• The kinetics molecular theory was developed to explain
the behavior of gases and to lend additional support to
the validity of the ideal gas law.
• Some of the important statements include:
1- Gases are composed of particles called molecules,
the total volume is so small as to be negligible in relation
to the volume of the space in which the molecules are
confined. This condition is approximated in actual gases
only at low pressures and high temperatures, in which
case the molecules of the gas are far apart.
Kinetic Molecular Theory
2- The particles of the gas do not attract one another but
rather quickly move with complete independence; again,
this statement only applies at low pressures.
3- The particles exhibit continuous random motion owing to
their kinetic energy.
4- The molecules exhibit perfect elasticity, that is, there is
no net loss of speed or transfer of energy after they
collide with one another and with the molecules in the
walls of the confining vessel, which latter effect accounts
for the gas pressure.
The Liquid State
.
• The liquid state is defined in comparison to the gaseous
and solid states:
• A liquid occupies a definite volume and takes the shape
of the container required to hold it.
• Liquids are denser than gases, are not compressible and
possess less kinetic energy than do gases.
• Liquids flow readily and the flow is influenced by friction.
• Liquids can be frozen, have boiling points and have
vapor pressure and surface tension.
The Liquid State
Liquification of Gases:
Cooling of the gas loss of kinetic energy reduction in
velocity.
Applying pressure intermolecular forces liquid state.
Transition from gas to liquid and from liquid to solid
depends on temperature and pressure.
Critical temperature: The temperature above which it is
impossible to liquefy a gas irrespective of the pressure
applied.
Critical Pressure: The pressure required to liquefy a gas at
its critical temperature. (Also it is the highest vapor
pressure a liquid can have).
The Liquid State
The critical temperature: serves as a rough measure for
the attractive forces:
E.g. Water: C.T. is 647°K; C.P. is 218 atm. {dipolar
forces, H-bonding}.
Helium: C.T. is 5.2°K; C.P. 2.26 atm {London Forces}.
Vapor Pressure of Liquids
• Molecules in the liquid state vary in the level of kinetic
energy they possess:
– Translational
– Vibrational
– Rotational
• When a liquid is placed in a container at a constant
temperature, molecules with the highest energy break
away from the liquid and pass into the gaseous state
(evaporate).
• On the other hand subsequently return to the liquid state
(condense).
Vapor Pressure of Liquids
• When the rate of evaporation is equal to the rate of
condensation at a definite temperature, an equilibrium is
established and the vapor is said to be saturated.
• Equilibrium vapor pressure is the pressure of a saturated
vapor above a liquid.
The effect of external pressure on the equilibrium vapor
pressure
Vapor Pressure of Liquids
• Factors affecting the vapor pressure:
– The nature of the attractive forces in the liquid e.g.
water < ethyl alcohol < diethyl ether.
– The effect of temperature: vapor pressure increases
with raising temperature.
• Not affecting: the vapor pressure of a liquid is
independent of the volume of the container, provided
that there is some liquid present so that equilibrium can
be established.
The liquid state-the Vapor pressure of
liquids
Vapor Pressure of Liquids
Raoult’s law:
• In an ideal solution, the partial vapor pressure of each
volatile constituent is equal to the vapor pressure of the
pure constituent multiplied by its mole fraction in the
solution.
PA= PA°XA
PB=PB°XB
• PA, PB: Partial vapor pressure of the constituents. XA, XB:
Molar fraction.
Heat of vaporization
• The relationship between the vapor pressure and the
temperature (absolute) of the liquid is expressed by the
Clausius-Clapeyron equation.
P2 H V (T2 T1 )
log
P1
2.303RT1T2
•
P1 and P2 are the vapor pressures at absolute
temperatures T1 and T2
• ΔHv is the molar heat of vaporaization (the heat
absorbed by 1 mole of liquid when it passes into the
vapor state).
Heat of vaporization
• ΔHv varies with temperature, however over a short range
it may be considered constant. For water at 100oC ΔHv is
539 cal/g while at 180oC it is 478 cal/g.
• The Clausius-Clapeyron equation can be written in a
more general form,
Hv 1
log P
cons tan t
2.303R T
or in natural logarithms,
ln P
H v 1
cons tan t
R T
from which it is observed that a plot of the logarithm of the
vapor pressure against the reciprocal of the absolute
temperature results in a straight line, enabling one to
compute the heat of vaporization of the liquid from the
slope of the line.
Heat of vaporization
• See example 2-7 page 29 – fourth edition (if you have
the fifth edition see example 2-7 page 33. If you have the
sixth edition see example 2-7 page 28).
• Solve problem 2-13 page 50 and problem 2-14 page 51
in the fourth edition (if you have the fifth edition solve
problems 2-13 and 2-14 page 686. If you have the sixth
edition solve problem 2-9).
Boiling Point
• If a liquid is placed in an open container and heated
until the vapor pressure equals the atmospheric
pressure, the vaporization process will be at its highest
rate.
• The vapor will form bubbles that rise rapidly through the
liquid and escape into the gaseous phase.
• The temperature at which this happens is called the
boiling point.
The boiling point is the temperature at which vapor
pressure of the liquid equals the external (atmospheric
pressure).
Boiling Point
• The boiling point may be considered as the temperature
at which thermal agitation overcomes the intermolecular
interactions of the molecules of the liquid.
• The boiling point of normal hydrocarbons, simple
alcohols and carboxylic acids increases with increased
molecule weight.
• Branching of the chain reduces the points of interaction
between molecules and reduces intermolecular
interactions and boiling point.
Boiling Point
Compound
Boiling Point
(oC)
ΔHv
(cal/g)
Helium
-268.9
6
Propane
-42.2
102
Methyl Chloride
-24.2
102
Butane
-0.4
92
Isobutane
-10.2
88
Ethyl ether
34.6
90
Ethyl alcohol
78.3
204
Water
100
539
Aerosols
• Gases can be liquefied under high pressure in closed
chambers as long as the chamber is maintained below
the critical temperature.
• When the pressure is reduced, the molecules expand
and the liquid reverts to gas.
• This reversible change of state is the basic principle
involved in the preparation of pharmaceutical aerosols.
Aerosols
• In an aerosol, a drug is dissolved or suspended in a
propellant.
• A propellant is a material that is liquid under the pressure
conditions existing inside the container but forms a gas
under normal atmospheric conditions.
• The container is designed so that by depressing the
valve, some of the drug-propellant mixture is expelled
because of the excess pressure inside the container.
Aerosols
• Propellants:
Florinated hydrocarbons: such as Trichloromonofluoromethane,
and dicholorodifluoromethane
Hydrocarbons: such as propane, isobutane.
Compressed gas: such as CO2, N2, and N2O
Aerosols
Aerosols
• If the drug is nonvolatile, it forms a fine spray as it leaves
the valve orifice, the liquid propellant vaporizes off.
• Chlorofluorocarbons (CFCs) and hydrofluorocarbons
(HFCs) have been most commonly used (Ozone
depletion).
• Alternate fluorocarbons propellants that do not deplete
the ozone layer of the atmosphere are under
investigation, in addition, an increase in the use of other
gases such as nitrogen and carbon dioxide is observed
during the last decade.
Aerosols
• The containers are filled either by:
– Cooling the propellant and drug to low temperature within the
chamber which is then sealed by the valve.
– Sealing the drug in the container at room temperature and then
forcing the required amount of the propellant into the container
under pressure.
• Aerosols are used for the delivery of antiseptic and local
anesthetics onto injured skin (ethylchloride effect !!).
• Exubera® inhaled insulin approved in 2006 by the FDA.
Vapor Pressure of Liquids
Calculate the vapor pressure at 298 K above an aerosol
mixture consisting of 30% w/w of aerosol propellant 114
(mol.wt = 170.9) with vapor pressure of 1.90x105 Nm-2
and 70% w/w of propellant 12 (mol.wt. =120.9) with a
vapor pressure of 5.85x105 Nm-2. Assume ideal behaviour.
Amount of a in mixture = 30/170.9=0.1755 moles
Amount of b in mixture = 70/120.9=0.5790 moles
Xa =0.1755/ (0.1755+0.5790)= 0.2326
Xb =0.5790/ (0.1755+0.5790)= 0.7674
P (total)=(1.90x105 x 0.2326)+(5.85x105 x 0.7674)
= 4.492x105 Nm-2
Solid State
• Solids are characterized as having a fixed shape and
being incompressible.
• Molecules in the solid state have strong intermolecular
forces and therefore very little kinetic energy.
• In solids there is very little translational and rotational
kinetic energy, however atoms vibrate around a fixed
equilibrium position.
• Very few solids are volatile enough to have a sublimation
point (nitroglycerin solid below 14oC).
Solid State
• Solids are usually characterized by:
–
–
–
–
–
–
–
–
Shape
Particle size
Melting point
Surface energy
Hardness
Elastic properties
Compaction
porosity
Solid State
• There are three main types of solids:
– Crystalline
– Amorphous
– Polymeric
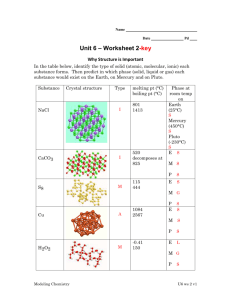
Crystalline Solids
• The molecules of a crystalline solid are arranged in
repetitious three-dimensional lattice units.
• Crystalline solids show definite melting points, passing
rather sharply from the solid to the liquid state.
• These three dimensional lattice units may assume
different shapes:
–
–
–
–
–
–
Cubic as in sodium chloride
Tetragonal as in urea
Hexagonal as in iodoform
Rhombic as in iodine
Monoclinic as in sucrose
Triclinic as in boric acid
Crystalline Solids
Crystalline Solids
• Different types of bonding may be involved in crystal formation.
Unit
Example
Bonding
Physical Characteristics
Atom to atom
Carbon, diamond,
graphite
Strong carbon
covalent bonds
Hard large crystals
Metallic
Silver
Strong metal bond
Positive ions in a field of free
moving electrons
Molecular
Menthol
Van der Waals
forces
Close packing, weakly held
together, low melting point
Ionic
NaCl
Electrostatic ionic
bonds
Hard, close packing, strongly
held together, high melting
point
Crystalline Solids
• Crystallization from solutions occurs as a result of three
successive procedures:
– Supersaturation of solution.
– Formation of crystal nuclei.
– Crystal growth round the nuclei.
Crystalline Solids
• Some materials may exist in more than one crystalline
form.
• Polymorphism is the property of having more than one
crystalline form.
• These different crystalline forms of the same material are
called polymorphs.
Crystalline Solids
• Polymorphs generally have different melting points,
stabilities, density, hardness, crystal shape, optical and
electrical properties, vapor pressure, and solubilities
even though they are chemically identical.
Crystalline Solids
• Crystalline solids may change its crystal system
reversibly with changes in temperature (enantiotropic
change)
• Changing the crystal form irreversibly is called a
monotropic change.
Crystalline Solids
• Theobroma oil (cocoa butter) is a triglyceride that is used
in pharmacy as a suppository base.
• Suppositories must be solid at room temperature but
melt at body temperature.
• The melting point of the most stable polymorph of cocoa
butter is approximately 35oC.
• If cocoa butter is overheated (>40oC) and cooled quickly
in the mold during the preparation of the suppository, it
will solidify in a less stable polymorphic form that melts
at lower temperature (15-28oC).
Crystalline Solids
• Many pharmaceutical solids are synthesized by an
organic chemical process, purified and then crystallized
out of different solvents.
• Residual solvents might get trapped in the crystalline
lattice, creating a cocrystal or a solvate.
• Solvates may be referred to as pseudopolymorphs.
Crystalline Solids
• A solvate may be defined as a molecular complex that
has incorporated the crystallization solvent molecules
into specific sites within the crystal lattice. When the
incorporated solvent is water the complex is called
Hydrates.
• Anhydrous compound, that doesn’t include water in its
structure.
• The hydrous and anhydrous forms of a drug differ greatly
in their solubility and melting points.
Crystalline Solids
Amorphous Solids
• These are solid materials where a long range order or
individual units is absent.
• Amorphous solids are referred to as glasses or
supercooled liquids because of the random order of
arrangement.
• Characterized by higher thermodynamic energy than
crystalline solids
• Usually the temperature of transition from an amorphous
solid into a liquid is a range of several degrees.
• The solubility of amorphous solids is more than that of
crystalline solids.
Amorphous Solids
• Prepared by:
– Rapid precipitation
– Lyophilization
– Rapid cooling of molten material
– Grinding
Amorphous Solids
• Anisotropy is the property of being directionally
dependent.
• Something which is anisotropic may appear different or
have different characteristics in different directions.
• An example is the double refraction of light coming
through a polarizing lens (Birefringence).
• Anisotropy is characteristic of most crystalline solids.
Amorphous Solids
• Isotropy is the property of being independent of direction.
• Isotropy is characteristic of cubic crystals and
amorphous materials.
Polymeric Solids
• Polymeric solids are made up of long chain molecules,
wrapped around each other. The polymer chains are
made up repeated monomers.
• Better flowability, elastic deformability.
• Polymeric materials may contain both crystalline and
amorphous domains.
Melting Point and Heat of Fusion
• The melting point or freezing point of a pure crystalline
substances: is the temperature at which the pure liquid
and solid exists in equilibrium.
• Usually it is reported as the temperature of the
equilibrium mixture at an external pressure of 1 atm
(Normal freezing point).
• Latent heat of Fusion: the heat absorbed when a gram of
solid change melts or the heat librated when it freezes.
Melting Point and Heat of Fusion
• The heat added during the melting process does not
bring about a change in temperature until all the solid
had disappeared.
Melting Point and Heat of Fusion
• Changes in the freezing point or the melting point with
pressure can be obtained using the following form of the
Clapeyron equation:
Vl Vs
T
T
P
H f
• Where Vl and Vs are the molar volumes (cm3/mole) of
the liquid and solid respectively.
• ΔHf is the molar heat of fusion.
• ΔT is the change in melting point brought about by a
pressure change of ΔP.
Melting Point and Heat of Fusion
• The molar volume is calculated by dividing the gram
molecular weight on the density of the compound.
Normal melting points and molar heats
of fusion of some compounds
Substance
Melting
Point K
ΔHf
H2O
273.15
1440
CH4
90.5
226
C2H6
90
683
n-C3H8
85.5
842
C6H6
278.5
2348
C10H8
353
4550
Melting Point and Heat of Fusion
• Water is unusual in that it has a larger molar volume in
the solid state than in the liquid state (VlVs) at the
melting point.
• Because of that, {∆T/∆P } is negative this means that the
melting point is lowered by an increase in pressure.
• This phenomenon is rationalized in terms of the Le
Chatelier’s Principle which states that a system at
equilibrium readjusts so as to reduce the effect of the
external stress.
Melting Point and Heat of Fusion
• Solve problem 2-18 part (b) page 51 in the fourth edition,
if you have the fifth edition solve problem 2-18 part (b)
page 687. If you have the sixth edition solve problem
2-12 part (b).
Melting Point and Heat of Fusion
• Heat of fusion: the heat required to increase the inter
atomic or intermolecular distances in crystals , thus
allowing melting to occur.
• Weaker bond---lower heat of fusion--- lower melting point.
– The melting point of saturated hydrocarbons increase with
molecular weight because of Van der Waal forces.
– Carbons of even no. of atoms has higher melting point than
those with odd no. of atoms ( odd no. has lower efficiency in
arrangement)
– Carboxylic acid: they crystallize more symmetrically than odd
ones. Reason: the carboxyl groups are joined at two points
compared to odd which are joined at one point
Melting Point and Heat of
Fusion
Melting Point and Heat of Fusion
• How to measure melting point:
– Capillary melting
– hot stage microscopy
– Differential scanning calorimetry
Characterization of the Solid State
• Thermals analysis (TA):
– Methods used to characterize the physical and
chemical alterations in material due to temperature
effect.
• TA is used for:
– Characterization and identification of drugs
– determination of purity
– Polymorphism
– solvent and moisture content
– stability and compatibility with excipients
Characterization of the Solid State
Characterization of the Solid State
• Heat absorbing (endothermic) processes:
– Fusion, boiling, sublimation, vaporization, desolvation, solid-solid
transition and chemical degradation.
• Heat generating (exothermic) processes:
– Crystallization and degradation.
• The major use of DSC for:
– Quantitative measurement for: purity, polymorphism, solvation,
degradation and excipients compatibility.
Characterization of the Solid State
• Thermogravimetric Analysis or TGA is a type of testing
that is performed on samples to determine changes in
weight in relation to change in temperature.
Characterization of the Solid State
• Vapor sorption/desorption analysis is a technique similar
to TGA.
• It measures weight changes in solids as they are
exposed to different solvent vapors and humidity and/or
temperature conditions.
• A positive change in the weight of the solid would
indicate that the solid material is absorping/adsorping
(sorping) the solvent.
Characterization of the Solid State
• The ability of the solid to continuously absorb water until
it goes into solution is called deliquescence.
• A weight loss could also be measured under low
relative humidity (desorption).
The Liquid Crystalline State
• It is a meso-phase (prefix meaning middle or
intermediate).
– Liquid-like: intermediate mobility and rotation.
– Crystal-like: being birefringent.
• Molecules that can form mesophase should be:
Organic, elongated and rectilinear in shape, rigid,
possess strong dipoles and easily polarizable groups.
• Liquid crystals are either thermotropic (the order of its
components is determined or changed by temperature)
or lyotropic (the ordering effects in it are induced by
changing its concentration within a solvent) .
The Liquid Crystalline State
• Types of liquid crystals: smectic, nematic and ,
cholestric.
• According to the shape of the crystalline structure.