Unit 6 Worksheet 2
advertisement

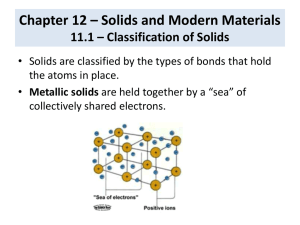
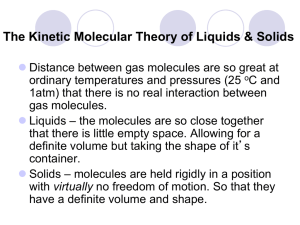
Name Date Pd Unit 6 – Worksheet 2-key Why Structure is Important In the table below, identify the type of solid (atomic, molecular, ionic) each substance forms. Then predict in which phase (solid, liquid or gas) each substance would exist on the Earth, on Mercury and on Pluto. Substance Crystal structure NaCl Type I melting pt (°C) boiling pt (°C) 801 1413 Phase at room temp on Earth (25°C) S Mercury (450°C) S Pluto (-230°C) S CaCO3 S8 Cu H2O2 I M 520 decomposes at 825 115 444 E M S P S E S M G A M 1084 2567 -0.41 150 P E S S M S P S E L M G P Modeling Chemistry S S U6 ws 2 v1 PbI2 CO2 Ar 402 954 -78 -57 -189 -186 E S M L P S E G M G P S E G M G P S Account for differences in the melting and boiling points of the three types of structures. Ionic solids generally have high melting and boiling points due to the strong bonds connecting the particles in a lattice. The molecules in a molecular solid are not as strongly attracted to one another and have lower melting and boiling points. In an atomic solid, if the atoms are not connected (as in Ar), the mp and bp are very low. In a metallic solid (like Cu), each atom is strongly attracted to all its neighbors, so these solids generally have high mp and bp. Predict which of these substances would conduct electricity when molten. Ionic solids form mobile charged particles when they melt, so they molten substance would conduct electricity. The electrons in metals are very loosely bound to the atoms, so a molten metal would also be a good conductor. Would any of these conduct electricity as a solid? Only metals conduct as solids, because the electrons are loosely bound to the atoms. Modeling Chemistry U6 ws 2 v1