The Kinetic Molecular Theory of Liquids & Solids
advertisement
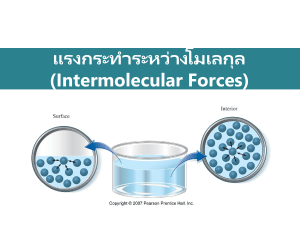
The Kinetic Molecular Theory of Liquids & Solids Distance between gas molecules are so great at ordinary temperatures and pressures (25 oC and 1atm) that there is no real interaction between gas molecules. Liquids – the molecules are so close together that there is little empty space. Allowing for a definite volume but taking the shape of it’s container. Solids – molecules are held rigidly in a position with virtually no freedom of motion. So that they have a definite volume and shape. Gases, Liquids, & Solids Intermolecular Forces These are attractive forces between molecules: Dispersion (London) Forces Dipole-Dipole Forces Hydrogen Bonding These forces are partly responsible for the nonideal gas law behavior discussed earlier. And these forces are why liquids and solids do not present “ideal behavior”. Keep in mind that intramolecular forces are forces within a molecule, and intermolecular forces are between molecules. Intermolecular Forces…………(cont.) Boiling points and melting points often reflect the strength of these intermolecular forces. Molecular substances tend to have the following characteristics: Non-conductors of electricity when pure, for example pure water and ethyl alcohol are 2 examples of molecular substances that, when pure, will not conduct electricity. Generally, are insoluble in water but soluble in non-polar solvents. They tend to have low melting and boiling points. The stronger the intermolecular force the higher the boiling or melting point. 1. Dispersion (London) Forces: The attractive forces that arise as a result of temporary dipoles induced in atoms or molecules. This happens when something (cation or polar compound) “throws off” the distribution of electrons in an atom or molecule. These forces can only occur in non-polar molecules. As molar mass (MM) increases the dispersion forces become stronger, consequently the boiling point of non-polar molecules tend to increase with MM. Dipole-Dipole 2. Dipole-Dipole Forces: These are attractive forces between polar molecules. The larger the dipole moment then the greater the dipole force. Polar molecules tend to have slightly higher boiling and melting points than non-polar substances of similar MM. Solids vs Liquids 3. Hydrogen Bonding: This a special, strong type of dipole-dipole interaction between hydrogen and polar bond in elements nitrogen, oxygen, and fluorine. Only between N—H, O—H, and F—H bonds have hydrogen bonding. These types of bonds tend to not follow the relationship between MM and boiling points. *It is important to remember that the 3 intermolecular forces mentioned above are relatively weak in comparison to the covalent bond within a molecule. Hydrogen Bonding Polarizability The ease with which the electron cloud of a particle can be distorted is called its polarizability. Smaller atoms (or ions) are less polarizable than larger ones because their electrons are closer to the nucleus and therefore are held more tightly. Thus, we observe several trends: Polarizability increases down a group because atomic size increases, so the larger electron clouds are farther from the nucleus and, thus, easier to distort and the greater the dispersion force. Polarizability decreases from left to right across a period because the increasing Zeff(effective nuclear charge) shrinks atomic size and holds the electrons more tightly and the weaker the dispersion force. Cations are less polarizable than their parent atoms because they are smaller; anions are more polarizable because they are larger. This concept also applies to nonpolar molecules such as H2, Example 1 Which of the following have the highest boiling point: a. Argon b. Neon c. Xenon d. Krypton Example 2 What types of intermolecular forces exist between the following pairs: a. Hydrogen bromide and hydrogen sulfide b. Diatomic chlorine and carbon tetrabromide c. Diatomic iodine and the nitrate ion d. ammonia and benzene Example 3 Would you expect to find hydrogen bonds in: Acetic acid Diethyl ether Hydrazine Example 4 What types of intermolecular forces are present in: nitrogen chloroform carbon dioxide ammonia water vapor and hydrogen gas Properties of Liquids Intermolecular forces give rise to a number of structural features and properties of liquids. Notable, two features: surface tension and viscosity. Surface Tension: the amount of energy required to stretch or increase the surface of a liquid be a unit area (for example 1 cm2). • Liquids that have strong intermolecular forces have high surface tensions. So because of hydrogen bonding, water has a greater surface tension than most other liquids. Viscosity: a measure of a fluids resistance to flow (unit = N * s/m2). • Liquids that have strong intermolecular forces have higher viscosities than those with weaker intermolecular forces. • Examples: Water = 1.01 x 10-3 Acetone = 4 x 10-3 Blood = 3.16 x 10-4 Glycerol= 1.49 Network Covalent Solids Most covalent solids are molecular however some form a network of repeating patterns, these are called network covalent solids. Plastics, and allotropes of carbon (diamond and graphite) do this. They tend to have high melting points compared to molecular covalent. Ionic Solids Consist of anions and cations that are held in a regular repeating arrangement by strong ionic bonds, or electrostatic interactions. Ionic solids tend to have high boiling and melting points. Ionic solids do not conduct electricity, because the ions are fixed. Many are soluble in water. Would solutions of ionic solids be good or poor conductors of electricity. The strength of the ionic bond depends on 2 things: The charge of the ion, CaO has a stronger ionic bond than NaCl The size of the ions: d = rcation + ranion Metallic Solids There is no real “bond” between metals. Basically the electrons of metals arrange around each other’s nuclei. This forms a “sea” of electrons, so that the electrons move about in the crystal. They are good conductors of electricity, have high thermal conductivity, tend to have luster (reflect light), are ductile and malleable, and are generally insoluble in water. Crystal Structures Ionic and atomic solids tend to crystallize in definite geometric forms. These geometric forms are made up of unit cells (the smallest structural unit in the 3-D repeating pattern). There are 14 possible crystalline structures but we will focus on the main 3. 3 Main Crystal Structures Simple Cubic (SC) Face Centered Cubic (FCC) Body Centered Cubic (BCC) How they differ… Number of atoms per unit cell SC FCC BCC The relation between side of cell, s (in nm), and the atomic radius, r. This relation is expressed in the following equation and offers and experimental way to determine the atomic radius of a metal: SC: 2r = s BCC: 4r = s1/3 FCC: 4r = s21/2 The percentage of empty space – the greater the amount of empty space the more unstable the structure. Example 5 Classify each of the following substances as atomic solid, molecular solid, ionic solid, or network covalent solid according to the type of solid it forms: Gold Carbon dioxide Lithium Fluoride Krypton Quartz (empirical formula SiO2) A Vapor A gas is a substance that is normally in the gaseous state at ordinary temperatures and pressures. A vapor is the gaseous form of a substance that is a liquid or a solid at normal temperature and pressure – generally 25oC and 1 atm. Vapor Pressure The pressure exerted by the vapor over the liquid remains constant (in a sealed container the rate of condensation becomes equal to the rate of evaporation). Diagram: Thus there is a state of dynamic equilibrium between the liquid and the vapor. So as long as the pressure exerted by the liquid is less than that of the atmosphere than the liquid will not boil. Diagram: Once the vapor pressure exerted by the liquid is equal to that of the atmosphere the liquid boils (this is considered the normal boiling point) Diagram: Vapor Pressure………………..(cont.) Vapor pressure always increases with temperature. The higher the average kinetic energy then the more collisions and the stronger the force exerted by the liquid (the vapor pressure). What pressure would be required to boil water at a temperature of 70*C if water exerts a pressure of 24mmHg? The stronger the intermolecular forces in the liquid the lower the vapor pressure of that liquid. Examples: Water – 24 mmHg Ether – 537 mmHg Why? The Clausius-Clapeyron Equation: T1 and P1 are a corresponding temperature (in Kelvin) and vapor pressure (in atm of mmHg) T2 and P2 are the corresponding temperature and pressure at another point ΔHvap is the molar enthalpy of vaporization (energy associated with vaporization) R is the gas constant (8.31 J mol−1K−1) This can be used to predict the temperature at a certain pressure, given the temperature at another pressure, or vice versa. Alternatively, if the corresponding temperature and pressure is known at two points, the enthalpy of vaporization can be determined. Example 6 Benzene has a vapor pressure of 183 mmHg at 40 oC. Taking its heat of vaporization to be 30.8 kJ/mol. Calculate its vapor pressure at 25 oC. Important Phase Change Terms Critical temperature (also called the critical point) – the temperature above which the liquid phase of a pure substance cannot exist. Basically, above this temperature the substance is a gas and below it the substance is a liquid. Critical pressure – conversely, this is the pressure that must be applied at the critical temperature to cause condensation. Supercritical Fluid – just means that the substance is above the critical temperature (refers to a gas). Normal Boiling Point – (of a liquid) is the temperature at which the vapor pressure of the liquid is exactly 1 atm. Melting point – pressure has little effect on melting point. Most solids are denser than their liquid form. There is a very important exception to the aforementioned, guess what it is? Triple Point Diagrams Heating Curve MC #’s 1-5 Use the following answers for questions 1 - 5. (A) A network solid with covalent bonding (B) A molecular solid with zero dipole moment (C) A molecular solid with hydrogen bonding (D) An ionic solid (E) A metallic solid 1. Solid ethyl alcohol, C2H5OH 2. Silicon dioxide, SiO2 3. Silver 4. Diamond 5. Carbon tetrachloride MC #’s 6-9 Use these answers for questions 6-9. (A) hydrogen bonding (B) hybridization (C) ionic bonding (D) resonance (E) van der Waals forces (London dispersion forces) 6. Is used to explain why iodine molecules are held together in the solid state 7. Is used to explain why the boiling point of HF is greater than the boiling point of HBr 8. Is used to explain the fact that the four bonds in methane are equivalent 9. Is used to explain the fact that the carbon-to-carbon bonds in benzene, C6H6, are identical MC #10 CH3CH2OH boils at 78 °C and CH3OCH3 boils at - 24 °C, although both compounds have the same composition. This difference in boiling points may be attributed to a difference in (A) molecular mass (B) density (C) specific heat (D) hydrogen bonding (E) heat of combustion MC #11 The melting point of MgO is higher than that of NaF. Explanations for this observation include which of the following? I. Mg2+ is more positively charged than Na+ II. O2¯ is more negatively charged than F¯ III. The O2¯ ion is smaller than the F¯ ion (A) II only (B) I and II only (C) I and III only (D) II and III only (E) I, II, and III MC #’s 12 - 14 Questions 12-14 refer to the phase diagram below of a pure substance. (A) Sublimation (B) Condensation (C) Solvation (D) Fusion (E) Freezing 12. If the temperature increases from 10° C to 60° C at a constant pressure of 0.4 atmosphere, which of the processes occurs? 13. If the temperature decreases from 110° C to 40° C at a constant pressure of 1.1 atmospheres, which of the processes occurs? 14. If the pressure increases from 0.5 to 1.5 atmospheres at a constant temperature of 50° C, which of the processes occurs? FRQ #1 The phase diagram for a pure substance is shown above. Use this diagram and your knowledge about changes of phase to answer the following questions. (a) What does point V represent? What characteristics are specific to the system only at point V?. (b) What does each point on the curve between V and W represent? (c) Describe the changes that the system undergoes as the temperature slowly increases from X to Y to Z at 1.0 atmosphere. (d) In a solid-liquid mixture of this substance, will the solid float or sink? Explain. FRQ #2 For each of the following, use appropriate chemical principles to explain the observation. (a) At room temperature, NH3 is a gas and H2O is a liquid, even though NH3 has a molar mass of 17 grams and H2O has a molar mass of 18 grams. (b) C (graphite) is used as a lubricant, whereas C (diamond) is used as an abrasive. (c) Pouring vinegar onto the white residue inside a kettle used for boiling water results in a fizzing/bubbling phenomenon.