Structure & bonding
advertisement

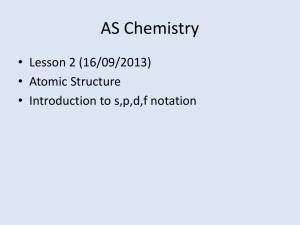
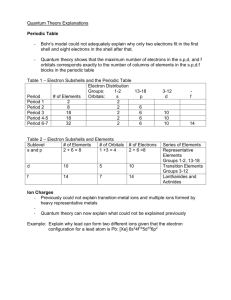
Windsor University School of Medicine STRUCTURE & BONDING A SOCIETY GROWS GREAT WHEN OLD MEN PLANT TREES IN WHOSE SHADE THEY KNOW THEY SHALL NEVER SIT. -- GREEK PROVERB Ch.5 J.C. Rowe LEARNING OBJECTIVES Bohr’s Theory of atomic structure is the beginning of quantum theory and describes electrons as residing in very particular "quantized" energy levels within the atom. Shells, Sub-shells and Orbitals are the backbone of the organizational structure of electrons in the atom Electron Configurations use shells, sub-shells and orbitals to describe the relative electronic organization within the atom. Periodicity of electron configurations relate to periodicity of physical and chemical properties. of valence shell configurations relate directly to relative reactivity. Bohr's Theory of the Atom and its structure Bohr found that: electrons move in particular 'orbits' within the atom-meaning electrons have particular places or levels within the atom, electrons have certain allowed energy values, the closer the electron is to the nucleus, the lower the energy when electrons are excited, they gain (or absorb) energy and move to a higher level (farther away from the nucleus), when electrons give off light, they emit energy and move to a lower level (closer to the nucleus), SHELLS, SUBSHELLS, ORBITALS Visualize the atom as an onion with different levels of structure and sub-structure, all of which make up electron energy levels – each major energy level is designated by a number (called the quantum number): Major Shell The major shell is the major layer of the onion--the large, thick peels which make you cry when you chop them. The major shells are designated by numbers which have integral values of 1, 2, 3, etc. These numbers are designated as the major shell quantum numbers, n. n = 1 designates the first major shell, the one closest to the nucleus and the one with the lowest (most negative) energy; n = 2 designates the second major shell, a little further from the nucleus; n = 3 designates the third major shell, etc. Major Shell Cont’d. The major shell is the primary influence on the energy of the electron. The electron "assumes" the energy of the major shell in which it resides. The closer the (negative) electron is to the (positive) nucleus, the stronger the attraction will be, and the harder the electron is to remove. So an electron in shell n=1 will be very hard to remove while an electron in shell n=4 would be much easier to remove. Major Shell Cont’d. Major shells contain subshells which are a substructure within the major shell. The number of the subshells is the same as the quantum number of the major shell, For example, major shell n = 1 has one subshell (specifically, an 's' subshell); n = 2 has two subshells (an 's' and a 'p' subshell), n = 3 has three subshells (s, p, and d subshells), and n = 4 has four subshells (s, p, d and f). Subshell The subshells are a substructure within the major shells-imagine each onion skin or peel containing another set of peels within each major peel The subshells are designated by letters s, p, d, f (these letters are terms which originated from the field of spectroscopy). s, p, d, and f are different types of subshells which have different properties which we will learn about soon. These subshells contain a further substructure, which are sets of orbitals which have the same energy. Orbitals Each subshell is further structured into orbitals. s subshells have one orbital p subshells have three, equal energy orbitals d subshells have five, equal energy orbitals f subshells have seven, equal energy orbitals each orbital can accommodate a maximum of two electrons, and Orbitals Cont’d each orbital takes on the characteristics of its subshell and major shell. s subshell orbitals (there is only one per major shell) are spherical, p subshell orbitals (there are three of them per major shell) are dumbell shaped and directed along the x, y and z axis, d subshell orbitals (there are five of them per major shell) have a different shape [I only want you to recognize s , p and d shapes] the relative energy levels and structure of the hydrogen atom What is the maximum number of electrons that can reside in the first major shell? In the diagram, the major shells are designated by the black lines coming from the energy arrow. The different types of subshells are indicated by the differently colored boxes. The orbitals themselves are the individual boxes. Remember, each box (orbital) can contain only a maximum of two electrons. Energies of the subshells Although the major shell primarily determines the energy of an electron, there are minor differences between the energies of the subshells within any major shell, particularly in an atom with more than one electron (actually, any atom other than hydrogen): s subshells are the lowest energy subshell within a shell p subshells are next lowest d subshells are higher than p subshells f subshells are higher than d subshells Orbital/Subshell energy levels Overlaps of subshells Once we have many electrons (which means many shells and subshells) in an atom, we begin to see some overlap of subshells--particularly, the s subshell from the fourth level (4s) overlaps with the d subshell from the third level (3d) such as shown above. Shapes of the subshells: For many reasons, we cannot define exactly where an electron is within an atom. We must, instead, define the volume which will most likely contain electron density. Consequently, the shapes of those volumes which can contain electron density are somewhat "fuzzy". High electron density vs. lower electron density. We can depict volumes of high electron density (many dots) or volumes of low electron density (few dots), as shown in the diagrams below. Orbitals An orbital is a region of high probability of finding an electron. There are different types of orbital : an (s) orbital with a spherical shape a (p) orbital with 2 egg-shaped lobes a (d) orbital with 4 egg-shaped lobes An (f) orbital with 8 lobes & more complex. Shape of (s) orbital Shape of (p) orbital Shape of (d) Orbital Types of Orbitals & Contents (s) orbital (p) orbital (d) orbital # orbitals 1 3 5 # electrons (maximum) 2 6 10 Subshells & shells Subshells grouped in shells. Each shell has a number called the principal quantum number, n The principal quantum of the shell is the number of the period or row of the periodic table where that shell begins Shells & subshells cont’d The principal quantum number(n) tells you how far an electron is from the nucleus The secondary quantum number (l) tells you what subshell (type of orbital) an electron is in. The magnetic quantum number (m or ml) tells you which orbital an electron is within a given subshell. Quantum Numbers Each orbital is a function of 3 quantum numbers n (major)……………….shell l (angular)………………subshell m.l (magnetic)……………orientation of electron within a subshell Quantum numbers cont’d symbol values description n (major) 1, 2, 3,……… Orbital size and Energy l (angular) 0,1,2,……n-1 Orbital shape or type m.l (magnetic) -l,….,0,….+l Orbital orientation Electron Configurations Pattern: 2 2 6 2 6 2 10 4p6 5s2 4d10 5p6 6s2 5d10 1s 2s 2p 3s 3p 4s 3d 6p6 lower energy higher energy strongest held e less tightly held e the letters refer to the type of subshell s, p, d or f the coefficients (numbers) to the left of each letter represent which major shell 1, 2, 3, etc. the subscript numbers represent the number of electrons present in the subshell. Pauli Principle & Hund’s Rule Pauli Exclusion Principle: an orbital can contain no more than two electrons and those two electrons must be paired, in other words, they must have opposite spin (usually indicated by one up-arrow and one down-arrow, as we will see shortly). Hund’s Rule: in a subshell where there are multiple orbitals with the same energy, electrons will enter then each orbital singly until all orbitals are half filled before pairing with other electrons in the subshell. Arrangement of electrons in atoms Each orbital can be assigned no more than 2 electrons. This is tied to the existence of a 4th quantum number, the electron spin quantum number, m.s Pauli Exclusion Principle states that No two electrons in the same atom can have the same set of 4 quantum numbers. That is, each electron has a unique address. Electron filling order (Aufbau order) 1 2 3 4 5 6 7 8 Aufbau order Electrons in Atoms When n=1, then l =n-1=1-1=0 This shell has a single orbital (1s) to which 2 electrons can be assigned. When n=2, then l= n-1= 2-1=1….. 0, 1 This shell has 2s orbital with 2 electrons Three 2p orbital with 6 electrons Thus a total of 8 electrons Electrons in atoms cont’d. When n=3, then l = n-1=3-1=2... 0, 1 ,2 3s orbital with 2 electrons Three 3p orbital with 6 electrons Five 3d orbital with 10 electrons Thus a total of 18 electrons Electrons in Atoms cont’d. When n=4, then l= 0, 1, 2, 3 4s orbital with 2 electrons Three 4p orbitals with 6 electrons Five 4d orbitals with 10 electrons Seven 4f orbital with 14 electrons Thus a total of 32 electrons Arrangement of electrons in atoms Electrons in atoms are arranged as Shells (n) Subshells (l) Orbitals (m.l) Periodic table vs. Aufbau order Valence electrons & shells Valence Electrons and Valence Shells: the most important electrons in an atom are those on the outer-most edge of the atom (the outer major shell) – they come into contact with other atoms first and participate fully in reactions – they are the valence electrons valence shells contain the valence electrons Periodic Table Pattern s-block Group IA and IIA valence electrons are in the ns subshell where 'n' denotes the major shell or row p-block Group IIIA - VIIIA valence electrons are in the ns and np subshell where 'n' denotes the major shell or row d-block Group IB - VIIIB (Transition Metals) valence electrons are in the ns and (n-1)d subshell f-block Inner Transition Metals sometimes called the lanthanides & the actinides valence electrons are in the ns and (n-2)d subshell Electronic Configuration Bonding Ionic vs. covalent bonding. Ionic bonds. Covalent bonds. Hydrogen bonds vs. Van der Waals’s forces Electronegativity Allotropy. Ionic vs. Covalent bonds Ionic bonding results from the transfer of electrons from a metal to a nonmetal forming a positively charged metallic ion and a nonmetallic ion, which are then held together by ionic bond. Covalent bonding results from the sharing of electrons btwn 2 nonmetals, btwn a nonmetal & a metalloids Ionic bonds Ionic bond is a complete transfer of one or more electrons from one atom to another Covalent bond A covalent bond is a bond btwn two atoms in which the electrons are shared. 1. Single covalent bond 2. (one pair of electrons shared.) 3. Doube covalent bond 4. (two pairs of electrons shared) 5. Triple covalent bond 6. (three pairs of electrons shared) Forms of chemical bonds Ionic bonds Any compound made out of a metal & a nonmetal is always an ionic compound and is held by ionic bonds. Covalent bonds 1. 2. 3. Normally forms btwn A nonmetal & a nonmetal A nonmetal & a metalloid A metalloid & a metalloid Ionic vs. Covalent Compounds ionic Crystalline Solid Conduct electricity when molten Variable H2O soluble High Melting pt, boiling pt, heat of fusion, heat of vaporization Tend to be very reactive Covalent Variable state Do not conduct electricity when molten Like dissolve like Low melting, boiling pt & low heat of vaporization, low heat of fusion Not as reactive Hydrogen bonds vs. Van der Waals’forces Hydrogen bonds Whenever a hydrogen atom is sandwiched in space between 2 oxygen atoms, hydrogen bond is made to hold the 3 atoms together. Van der Waals’ forces Exist btwn atoms & molecules but this bond is neither a covalent nor ionic bond. It is a weak bond Electronegativity Electronegativity is the ability of an atom in a molecule to attract shared electrons to itself. Metals have a low electronegativity. Nonmetals have a high electronegativity. Fluorine is the most electronegative element; it pulls very strongly on the shared electrons in a covalent bond. Electronegativity Cont’d. Excluding the noble gases, elements that are closer to fluorine have a higher electronegativity than elements that are further away from fluorine. The greater the difference in electronegativity btwn two elements that are bonded to each other, the more polar that bond is. If the electrons are shared equally, the bond is a nonpolar covalent bond. If the electrons are not shared equally, then the bond is a polar covalent (meaning that the electrons spend more of their time around one atom than the other). Allotropy Elements whose atoms bond together in more than one way exist as allotropes. Because the properties of a substance are partly set by its structure, the allotropes of an element usually have different appearances, melting point and boiling points, and electrical properties. E.g. Carbon has 3 allotropes : diamond, graphite and fullerene. Diamond vs. Graphite Diamond vs Graphite Allotropes Fullerene allotrope Any carbon constituted molecule in the form of a hollow sphere, ellipsoid, tube etc shape. Detected in nature & outer-space Important for the future of nanotechnology & electronics + cancer + HIV research André Gide One doesn't discover new lands without consenting to lose sight of the shore for a very long time.