Written Part of PS IX
advertisement

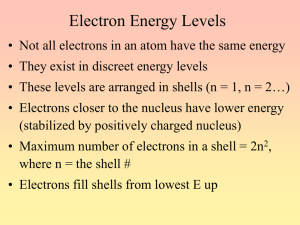
Name: Period: Problem Set IX Due Beginning of Class Fri May 15, 2009 Rules: This problem set consists of two parts. You will find several questions on Webassign for you to answer. In addition, you must answer the questions below neatly, and in order, on a separate sheet of paper. You may work together on this project, or ask Mr. Price questions. You must show all work for full credit. 1) Place the following types of radiation in order of increasing energy per photon a) yellow light from a sodium lamp b) x-rays from an instrument in a dentist’s office c) microwaves in a microwave oven d) you favorite FM music station at 91.7 MHz 2) Draw pictures of the: a) 1s orbital b) 2s orbital c) 2p orbitals 3)How many orbitals exist for the: a) 1s subshell b) 3s subshell c) 3p subshell d) 6d subshell 4)How many total orbitals and electrons can there be in the (show your work): a) n=1 level d) 4s sublevel b) 2p sublevel e) n=2 level c) 5d sublevel 5)Write complete electron configurations for the following atoms: a) Sc b) P c) C d) Zn e) Br 6)Write noble gas configurations for the following atoms a) K b) Ar c) Ge d) B 7)Draw orbital diagrams for the following atoms: a) The lightest halogen atom b) The second lightest alkaline earth metal c) The noble gas the follows bromine 8)Identify the following elements a) The ground state configuration is [Ne] 3s23p4 b) The ground state electron configuration contains three unpaired 6p electrons c) An excited state of this element has the electron configuration 1s22s22p53s1 9)A certain oxygen atom has the electron configuration 1s22s22px22py2. How many unpaired electrons are present? Is this an excited state of oxygen? In going from this state to the ground state would energy be released or absorbed? 10)How many unpaired electrons and how many valence electrons are in the ground states of a) K b) Ti c) Al d) Sc e) Br 11)On the blank periodic table, label the following: a) Alkali metals b) Alkaline earth metals c) Halogens d) Noble gases e) Transition metals f) Noble metals g) Representative elements h) Inner transition metals i) Actinides j) Lanthanides k) Metalloids 12)Consider the element Arsenic a. How many electrons does it have? b. What is its atomic number? c. Write down its complete electron configuration d. Write an orbital diagram for arsenic e. How many unpaired electrons does it have? f. What is its highest occupied energy level? g. How many core (inner shell) electrons does it contain? h. How many valence electrons does it have? i. What is the value of n for the valence shell?