Unique Properties of Water
advertisement

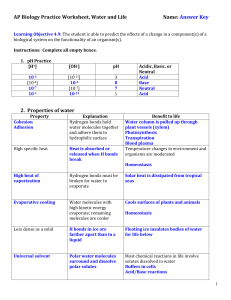
Title: Unique Properties of Water Introduction: All living things are dependent on water. Your cells are mostly water and they are surrounded mostly by water. Approximately 70% of the planet is covered by water though most of it is salty and not drinkable 1- Structure of water and attraction Structure of water Water has two hydrogen atoms each joined to an oxygen atom by a single covalent bond Water is a polar molecule. This means that it has an unequal charge. The hydrogen’s get pulled toward the oxygen Attraction Water molecules are attracted to each other. The slight negative charge at the oxygen end attracts the slightly positive ends at the hydrogens. The bonds between these water molecules are called hydrogen bonds 2- Waters life Supporting Properties Cohesion Cohesion is the tendency of molecules to stick to other molecules of the same kind. Cohesion is much stronger in water than in other liquids Adhesion Adhesion is the tendency of water molecules to stick to other types of molecules Why is this important? This is important for several reasons - Adhesion and cohesion help to keep large molecules organized and operational in the cell Transpiration – As water molecules are lost in leaves during photosynthesis a partial vacuum is formed in the vessels of the plant that carries water. Adhesion and cohesion allow water to travel against gravity and go from the roots to the tips if the highest trees Temperature Moderation Hydrogen bonds help water to resist temperature changes. Think boiling water. Which heats up faster the water or the pot? - Low density Thermal energy – The total amount of energy associated with the random movement of atoms in matter Temperature – is the average energy of the random movement When two substances differ in temperature heat is transferred from the warmer object to the cooler object (when you go into a pool you get cold because you lose body heat into the cooler pool) Density is the amount of matter in a given volume of space. Density = mass / volume As materials become solid the material in them tends to become more compact – more dense. However this does not occur in water. As water becomes a solid the hydrogen bonds keep the molecules from becoming tightly packed. Hence, ice becomes less dense than liquid water – This allows ice to float Universal Solvent - Why is this so important to life on Earth? A solution is a uniform mixture of two or more substances, i.e., salt and water or lemonade and water - The substance that does the dissolving is called the solvent The substance that is dissolved is called the solute When water is the solvent it is called an “aqueous solution” Water is the main solvent in our cells Water is considered the universal solvent because many things dissolve in it. Including virtually everything in our cells, allowing reactions to occur, all ionic and many non-ionic compounds