Property of water definition surface tension Cohesion Adhesion
advertisement
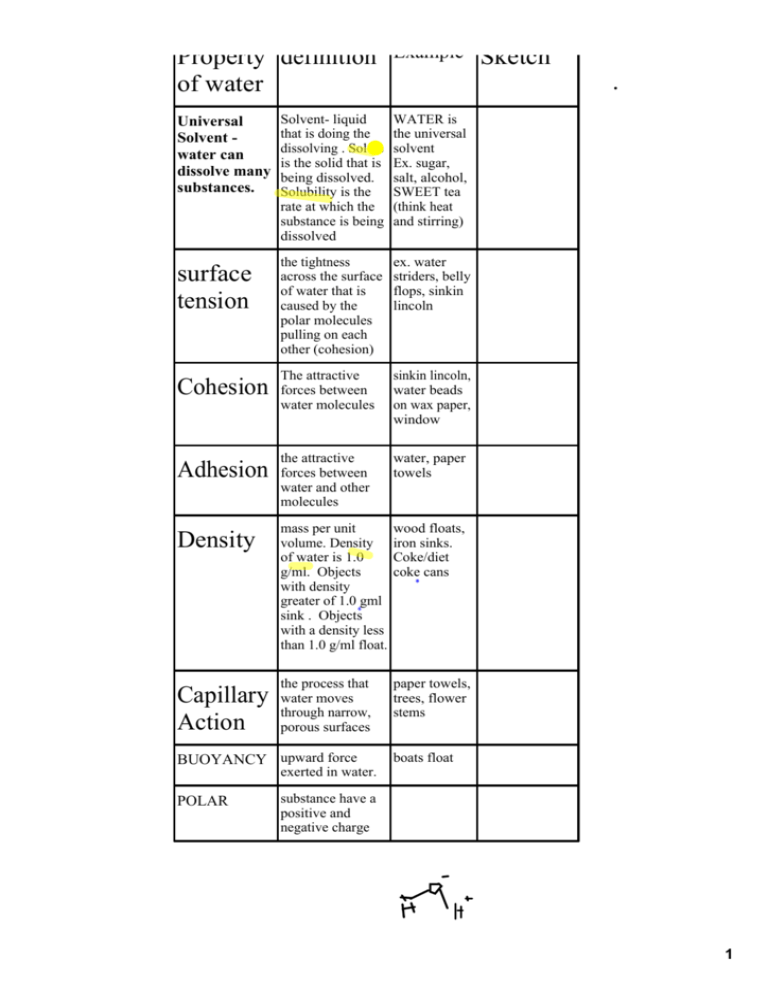
Property definition of water Universal Solvent ­ water can dissolve many substances. surface tension Example Solvent­ liquid that is doing the dissolving . Solute is the solid that is being dissolved. Solubility is the rate at which the substance is being dissolved WATER is the universal solvent Ex. sugar, salt, alcohol, SWEET tea (think heat and stirring) the tightness across the surface of water that is caused by the polar molecules pulling on each other (cohesion) ex. water striders, belly flops, sinkin lincoln The attractive sinkin lincoln, water beads on wax paper, window Cohesion forces between water molecules the attractive Adhesion forces between Sketch water, paper towels water and other molecules Density mass per unit volume. Density of water is 1.0 g/ml. Objects with density greater of 1.0 gml sink . Objects with a density less than 1.0 g/ml float. wood floats, iron sinks. Coke/diet coke cans the process that paper towels, trees, flower stems Capillary water moves through narrow, Action porous surfaces BUOYANCY upward force boats float exerted in water. POLAR substance have a positive and negative charge 1 PROPERT Y OF WATER DEFINITION EXAMPLE SKETCH POLAR COHESION ADHESION SURFACE TENSION UNIVERSAL SOLVENT BUOYANCY DENSITY 2