Absolute Age Dating
advertisement

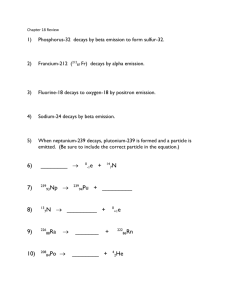
Geologic Time and Absolute Dating Review: Atomic Structure • Atom – Basic unit of an element – Composed of protons and neutrons (nucleus) surrounded by electrons – The identity of an atom is determined by the number of protons the atom has Example: Krypton • Krypton’s atomic number is 36, therefore – A neutral krypton atom has 36 protons and 36 electrons – If atomic number ≠ 36 ≠ krypton – The number of neutrons =atomic number-atomic weight (rounded up)=48 – The number of neutrons can vary without altering the identity of an atom—Isotopes Isotopes Stable… • Isotopes are like people—some are stable; some are not • And, like people, it’s the unstable ones that attract our attention the most Seriously unstable… Radioactivity • Unstable isotopes are radioactive—their nuclei will decay over time • A any radioactive isotope is called a “parent” isotope • The decay product is called the “daughter” isotope • When an isotope decays, they do so in one of three ways… • Alpha emission – Nucleus emits two protons and two neutrons • Plutonium-240 decays to uranium-236 • Beta emission – Nucleus emits an electron • Radium-228 decays to Actinium-228 • Electron capture – An atom’s nucleus captures an electron which reacts with a proton creating a neutron • Carbon-11 decays to Boron-11 • In a nutshell: When the nucleus decays, a new, more stable isotope is created Electron capture Radioactive Decay and Popcorn…yummy • Radioactive decay is a spontaneous and irreversible process • Ex. popcorn If the Decay of an Atom Occurs Randomly, How is it Useful to Us? Sample of actinium Even a small sample is composed of billions of actinium atoms (Ac-227) After 22 years, exactly half of the Atoms have decayed to thorium-227 All isotopes of actinium are unstable and will decay over time. Since every atom has a certain probability of decaying, on average, half of the atoms in a given sample will decay to a (more) stable daughter isotope over a set period of time Actinium-227 has a half-life of 22 years Half-lives • We can use the half-life of an isotope to figure out the age of a rock • How can we do this? – Half-lives are constant • Actinium-227 always has a half-life of 22 years – As the parent decays the daughter accumulates • Older samples = higher number of daughter isotopes Example • If we have a rock with 100 grams of a particular isotope (Bob-12) • Bob-12 decays to Joe-11 and has a half-life of 400 Million years • How old is our sample if only 25 grams of Bob-12 remain? – Our sample is 800 My old 100 grams of Bob-12 400 My (one half-life) 50 grams 50 grams + Bob-12 Joe-11 = 100 g 400 My years (now two half-lives have passed) 25 grams 75 grams + = 100 g Bob-12 Joe-11 Good vs. Bad • Isotopes with long half-lives are good for old rocks • Young materials are best dated by short lived isotopes Commonly Used Isotopes