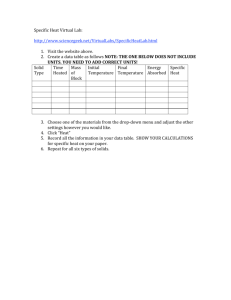
Solutions Review

Thermochemistry 5
Boon Chemistry February 6, 2013
Catalyst
(1)
What formula would you use to calculate the heat needed to increase the temperature of the ice from 200 K to 273 K?
(2) What formula would you use to calculate the heat needed to melt the ice?
Objectives
• I can solve for the total heat transferred to heat or cool a sample through a phase change.
Agenda
Catalyst
Multi-Step Thermochemistry
Problems
Practice Calculations
Multi-Step Thermochemistry Problems
Today we are calculating the amount of heat transferred to increase or decrease the temperature of a substance and cause a phase change.
Example: How much heat is absorbed when 100 g of water is heated from 365 K to steam at
400 K?
We are combining what we learned last week and this week.
Multi-Step Thermochemistry Problems
Step 1: Break down the problem into “temperature change” and “phase change” steps.
Use the boiling point and melting point as a guide.
Step 2: Solve the steps separately.
Use q=mc Δ T for temperature change and q = m Δ H t change for phase
Step 3: Add up the answers to get the total heat transferred.
Example: How much heat is absorbed when 100 g of water is heated from 365 K to steam at 400 K?
Step 1: The boiling point of water is 373 K. So, break up the problem in three parts: (a) 365K to 373K, (b) vaporization and (c) 373K to 400K.
Step 2: Set up and solve the problems. Then step 3: add up the answers.
Example 2: How much heat is released when 2500 g of water is cooled from 70 °C to ice at -10°C?
Step 1: The melting point of water is 0°C. So, break up the problem in three parts: (a) 70°C to 0°C, (b) freezing and (c) 0°C to -10 °C.
Step 2: Set up and solve the problems. Then step 3: add up the answers.
Think, Pair, Share
How many steps would you split these problem into?
Why?
(1)
How much heat is absorbed when 5000 g of water is heated from 275 K to 370 K?
(2)
How much heat is absorbed when 10,000 g of ice at
0°C is melted and heated to steam at 150°C?
Work Time
1. Work quietly at your table to complete the practice problems.
Get a stamp when you finish and check your work.
2. Work on any thermochemistry worksheets that you have not yet finished.
Homework
Due Thurs/Fri (Feb. 7 & 8): All Thermochemistry notes and handouts.