Study Guide Chapter 6 - Thermochemistry 1. Definition of potential
advertisement

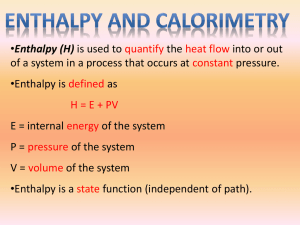
Study Guide Chapter 6 - Thermochemistry 1. Definition of potential and kinetic energy 2. What is heat, temperature, how are they different 3. Definition of Work W=force x distance A. Definition of Work when only changed of pressure or volume are allowed W= -P x change in volume 4. What is a state function 5. Define exothermic endothermic, exogonic endogonic 6. Define internal energy, enthalpy what is the difference? )E=q + w )H = E + )PV 7.How do we measure? )E=q v ie. Heat when vol is kept constant (bomb calorimeter) )H = qp ie Heat when pressure is kept constant (simple calorimeter) 8. Use the equation )E = q + w in problems, using proper sign convention 9. Define heat capacity and use in various calculations 10. How is )H defined for a chemical reaction ()Hrxn = )Hproducts - )Hreactants 11. What is Hess’s law? - use it in different problems 12. What is a standard enthapy of formation? A. What is the standard state for a given compounds B. Use standard enthapies in various problems to calculate )H of a reaction