Chapter 2 - The Chemical Basis of Life
advertisement
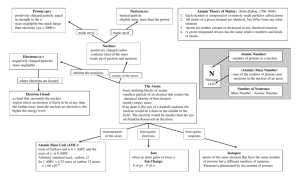
Chapter 2 - The Chemical Basis of Life NEW AIM: What’s the matter? Figure 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What is MATTER ? - Anything that takes up space and has mass Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? The smallest chemically unbreakable unit of MATTER ? An atom Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Fig. 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Fig. 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Fig. 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Fig. 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Elements - The various types of atoms Ex. Carbon, Oxygen, Nitrogen, etc… Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? “Minerals” Table 2.1 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Fig 2.3b Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? A lack of iodine in one’s diet can cause swelling of the thyroid gland resulting in a GOITER. The condition is reversible if iodine is taken. (Don’t worry, we iodize salt) Fig 2.3b Iodine is used by thyroid cells to make hormones (chemicals released by one cell into the blood and bind to a receptor on another cell, which is one way cells talk to each other). Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Element vs Compound (Emergent Properties) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Elements – composed of the same types of atoms Compounds – composed of two or more types of atoms Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Element - Any substance composed of only ONE element Ex) a bar of pure gold, nitrogen gas (N2), oxygen gas (O2) Compound - Any substance composed of two or more elements Ex) Na+Cl- (table salt), H2O, CO2 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Pure sodium (element) Chlorine (Cl2) gas (element) Na+Cl- Table Salt (Compound) + Fig 2.2 = EMERGENT PROPERTIES (EP’s) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? The forces and energy Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What are atoms made of and how are they organized Niels Bohr Danish Physicist 1885-1962 (The Bohr Model) ? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Nucleus Organization: Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Subatomic particles (sub = below, below the atom level) proton neutron electron charge mass +1 1 amu (dalton) 1 amu (dalton) 0 -1 amu = atomic mass unit 1 amu or 1 dalton = 1.67 x 10-27 kg 1/1836th an amu (dalton) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? How are the electrons “held” to the nucleus? Why do they not just shoot away? Electrons are held to the nucleus by the electromagnetic (EM) force since electrons are negative and the nucleus is positive – opposite charges attract / like charges repel. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? How are the protons “held” together in the nucleus? Why do they not break apart due to the EM force? The strong force holds the nucleus together. The strong force is only “felt” at extremely small distances, which is why the electrons do not feel it. You would need to be on the nucleus to feel it. For example, if gravity were like this, you would only feel it on Earth, but if you jumped up a few feet, you would no longer be pulled down by it... Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Electron Organization: n=1 n=2 Electrons are present in shells and move around the nucleus at a speed of ~2200 km/s. They can only be in these shells and nowhere else!! The first shell (n=1) can hold up to 2 electrons. That means it can have 0, 1 or 2 electrons in it at any time. The second shell (n=2) can hold up to 8 electrons. The third shell (not shown, n=3) can also hold up to 18 electrons. There are many more shells, but you only need to know the first three for AP Bio. 2n2 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What is the current model of the atom? (The Quantum Model) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Electron Organization: Which shell contains higher energy electrons, shell 1 (n=1) or shell 2 (n=2)? Explain. n=1 Shell 2. The further from the nucleus the electron, the further it can fall toward the nucleus and therefore it has more energy (a greater ability to accelerate matter) than shell 1 electrons. n=2 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Electron Organization: Electrons can jump between shells (called a quantum leap) n=1 In order to get an electron to “leap” from n=1 to n=2, what is required? n=2 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Electron Organization: Electrons can jump between shells (called s quantum leap) n=3 n=2 n=1 Fig 2.7 The nucleus is charged and therefore is pulling on the electron. It will take energy to pull the electron away from the nucleus and move it further away to shell 2. This is analogous to picking up a bowling ball. Earth is pulling on the bowling ball like the nucleus is pulling on the electron. It takes energy to pick up a bowling ball. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Electron Organization: Electrons can jump between shells (called s quantum leap) What occurs when an electron leaps in the opposite direction from n=2 to n=1? Energy is emitted or lost from the electron (in the form of light). n=3 n=2 n=1 Fig 2.7 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? How big are atoms? 1/100,000,000,000th mm 0.62 miles . Analogy: If the nucleus were enlarged to the size of a golf ball, the first electron shell would be over ½ mile away. What does this tell you about the composition of an atom or matter in general as we know it on Earth? Matter as we know it is 99.999999999% empty space and therefore has an extremely low density. 1/10,000,000th mm (size) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Then what is matter at the microscopic level? Mostly empty space with tiny spaced out dots of matter (protons/neutrons/electrons) held together by the electromagnetic and strong force. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? If we are just a collection of dots, why can’t we pass through each other? Why don’t I just fall through the floor? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Quizicule 1. Identify the four forces of the Universe. 2. What is a force? 3. What does the term “energy” imply? 4. What force do we as humans use when we apply force to objects? 5. Why do we rely of the force of gravity in order to make our bodies move around? Be brief. 6. If a nucleus of an atom were scaled to the size of a golf ball, how far away would the first electron need to be placed approximately? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? A mole (Avogadro’s Number) 602,300,000,000,000,000,000,000 or 6.023 x 10 Aside: Make sure you know scientific notation and unit conversions… 23 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? A mole of money spent at 1 billion dollars per 602,300,000,000,000,000,000,000 second would take 19ormillion23years to spend… 6.023 x 10 (scientific notation) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? A mole (Avogadro’s Number) 602,300,000,000,000,000,000,000 or 6.023 x 10 WHY? 23 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Mass (amu) If you have a mole… Mass (grams) conclusion 1 proton (1 amu) X 6.02x1023 = 1 gram 1 mole of protons has a mass of 1 gram (1 amu…1 gram) 1 neutron (1 amu) X 6.02x1023 = 1 gram 1 mole of neutrons has a mass of 1 gram (protons and neutrons have essentially the same mass) 2 protons (2 amu) X 6.02x1023 = 2 grams If one mole of protons is one gram then a mole of 2 protons will be 2 grams (2 amu…2 grams) 2 protons and 2 neutrons (4 amu) X 6.02x1023 = 4 grams I hope you are seeing it now… (4 amu…4 grams) Carbon-12 atom X 6.02x1023 = (1 mole) (1 mole) (1 mole) (1 mole) 12 grams Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? You learned in chemistry that if you know the amu of a substance then you know the number of grams/mole (gram formula mass). You should not be able to explain why this works so nicely…explain (size) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? How many water molecules are in 18ml of water (size) ? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Quizicule 1. How many marbles do I have if I have 2 moles of marbles? 2. What is the significance of a mole? (Why is a mole defined as 6.02e23? 3. Identify the two forces at play within the nucleus. 4. If the nucleus were scaled to the size of a golf ball, approximately how far away would the first electron be? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Questions 1. How many grams of KCl would you need to weigh out in order to have 1 mol of KCl’s? 2. If I have 180g of glucose, how many moles of glucose do I have? How many glucose molecules do I have? 3. How many ml of water would I need in order to have 3 moles of water molecules? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What determines the elemental identity of an atom Explain why. ? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Always has 2 protons Always has 6 protons Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Memorize the first 20 – Hydrogen to Calcium Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What determines the elemental identity of an atom ? The number of protons = atomic number (Z) Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Element? Mass? Charge? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? The atomic mass (A) = protons + neutrons Why do we not add the electrons mass? Electrons are soooo small relative to protons and neutrons (1/2000th the size) that we ignore them. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? The atomic charge Compare number of protons to the number of electrons. Ex. If there are 10 protons (+10) and 7 electrons (-7) the overall charge is +3 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What happens if we change the number of protons ? You change the identity of the atom (becomes a different element) as well as the mass. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What happens if we change the number of neutrons You change the mass and perhaps the stability.... ? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Isotopes are atoms that have the same elemental identity (same number of protons/same properties), but different number of neutrons. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? stable stable unstable Certain ratios of protons to neutrons are unstable resulting in breakdown of the nucleus (nuclear radiation). Ex. 6 protons and 6 neutrons in a nucleus (Carbon-12) is stable, but 6 protons and 8 neutrons (carbon-14) is unstable and will undergo radioactive decay to become stable. C-14 is called a radioactive isotope. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Radioactive decay Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Radioactive decay Carbon-14 (radioactive) will decay to Nitrogen-14 (stable). Seven protons and 7 neutrons in a nucleus is stable. An electron is shot out during the decay making it dangerous and useful. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? How can we use radioactivity to our advantage ? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? At what temperature is DNA replication most efficient in humans? Example: 3H (tritium) = radioactive isotope of hydrogen. How many protons and neutrons? 1 proton, 2 neutrons 1. Add tritiated thymidine (thymidine with 3H hydrogens) to human cells undergoing mitosis in petri dishes. (tritiated thymidine will be converted to the nucleotide dTTP (T) and used to make DNA) 2. Place dishes at different temperatures for a couple hours. 3. Remove dishes, put cells in test tubes and centrifuge down cells and measure radioactivity. What would you predict to observe? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Example: 3H (tritium) = radioactive isotope of hydrogen. How many protons and neutrons? 1 proton, 2 neutrons What would you predict to observe? Cells growing at 37C performed DNA replication the quickest as human enzymes typically work best at body temperature. Therefore these cells with have the greatest amount of radioactivity. Cell at greater than 50C or less than 20C will have little to no radioactivity as DNA replication enzymes will not work at these temps. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Tumor (mass of unregulated dividing cells) with concentrated radioactive glucose. PET scan We can send molecules like fluorodeoxyglucose (FDG) (glucose with radioactive fluorine (F-18) in place of a hydroxyl (-OH) group) containing radioactive elements into your body. For example, cancer cells use lots of glucose and therefore the glucose will build up where tumors are found. The radioactive elements will be shooting out particles (electrons or others) like a billion microscopic flare guns that we can detect (in this case using PET scanning). Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Tumor (mass of unregulated dividing cells) with concentrated radioactive glucose. We can potentially trace (follow) any radioactive molecule we put into you. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What if we alter the ? number of electrons If you alter the electrons, you simply change the charge of the atom Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Quizicule 1. What determines the elemental identity of an atom? 2. Why is a mole equal to 6.02e23? 3. Draw the bohr model of a carbon atom with a charge of -1 in its lowest energy state. 4. Atoms of the same elemental identity, but differ in their number of neutrons are known as __________________. 5. If sucrose has a total of 342 protons and neutrons of which 182 are protons , and I have 182g of sucrose, how many moles of sucrose am I sporting? 6. How many grams of sodium bicarbonate (NaHCO3) would I need to mass out if I wanted to have 1.5 moles in my possession? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Review: 1. Draw an oxygen atom with a charge of -2 and an atomic mass of 18 2. What is the atomic number of this atom? 3. Draw a neutral isotope of the above atom. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Review: 4. How many protons, neutrons and electrons does the above element contain? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Review: 5. Which of the following models is correct according to the Bohr model of the atom? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Review: 6. You have an atom and determine its mass to be 150 amu and to have 90 neutrons. The atom has an overall charge of -3. How many electrons does the atom have? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Review: 7. What is a mole? Why is this number important? From where did it originate? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? What determines the ? Chemical reactivity of a given atom (how an atom will interact with another atom) The number of electrons in the valence shell (outer shell). This is logical as you would expect the outer surfaces of atoms to interact with each other. Of course, the number of protons determines the number of electrons and therefore it boils down to the number of protons in the nucleus, which is why protons determine the element. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Valence shell Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Climbing the Hierarchy Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? When are atoms most happy ? When their valence shell is full. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? To be “happy” (stable) the sodium atom will need to either get 7 electrons or lose 1. Which is easier? The sodium will give away its outer shell electron. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chlorine needs one electron for its outer shell to be full. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chlorine has a higher AFFINITY for the electron and therefore the electron will “fall” from sodium to chlorine. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? When the piece of elemental sodium (countless numbers of sodium molecules) is placed in the chlorine gas in the video, all those ridiculous number of electrons jump to the countless chlorines. This causes all the molecules to move around violently (heat up) and electrons to jump between shells (give off light). Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Ionic bond When sodium loses an electron it becomes positively charged (a cation). When chlorine picks it up it becomes negatively charged (a anion). Cations and Anions are collectively called ions = fully charged atoms/molecules. ions Cation vs Anion Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Ionic bond The sodium and chloride ions are now attracted to each other and form an ionic bond. ionic bond = bond between two oppositely charged ions ions Cation vs Anion Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Salt crystals Na+Cl- crystals are repeating arrays of Na+ and Clheld together by the electromagnetic force. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Salt -general name given to ANY ionic compound (not just sodium chloride (Na+Cl-) held together in a lattice structure. Na+ClK+ClMg2+Cl2Mn2+Cl2Ca2+CO32- Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? How else can atoms fill their outer shells? http://www.visionlearning.com/library/flash_viewer.php?oid=1348&mid=55 Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Single Covalent Bond H H or H2 In this case, to be stable and fill their outer shells, unlike in an ionic bond, the atoms will SHARE their electrons to form a covalent bond. http://www.visionlearning.com/library/flash_viewer.php?oid=1348&mid=55 Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? F F or F2 Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Double Covalent Bonds (double bond) = = O C O or CO2 Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Draw : CH4 or H2O Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Why is oxygen gas O2 and nitrogen gas N2 = O O and = N -N Covalent bond strengths: Triple bond > Double bond > Single bond ? Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? Fig. 2.11 Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? MOLECULE A molecule is two or more atoms held together by COVALENT bonds. If you see a covalent bond, it is a molecule. Period. The End. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? C9H8O4 = Molecular formula Structural formula: skeletal formula An aspirin molecule Displayed formula Ball-and-stick Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? C9H8O4 = Molecular formula Why is the molecular formula not usually enough to tell us what molecule is being indicated? Because the atoms can be attached in different ways and different structures will give us different molecules with different functions called structural isomers or constitutional isomers. Chapter 2 - The Chemical Basis of Life AIM: How do atoms interact with each other? An antibody molecule (a type or protein) compared to an aspirin molecule (the smaller one). Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Drawing molecules 1. Lewis-dot diagram (valence) 2. Display 3. Skeletal Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Draw the structure of C2H6 HH HCCH HH Draw the structure of H2CO H-C=O H Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Draw the structure of H6C3O Draw the structure of C6H6 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Number of bonds formed by each element: Element Number of Covalent Bonds it will form to be “happy” (stable) Hydrogen 1 2 3 Oxygen 4 Nitrogen Carbon The element will form as many bonds as it needs electrons in its valence shell. For example, carbon needs four electrons and therefore it will make four covalent bonds (it will share 4 electrons) with other elements. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Using the terms element, compound and/or molecule, characterize the following: C6H6 This is certainly a molecule because there will be covalent bonds. It is also a compound because there is more than one type of element. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Using the terms element, compound and/or molecule, characterize the following: + Na Cl This is not a molecule since there are no covalent bonds present. It is a compound since there is more than one type of atom present. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Using the terms element, compound and/or molecule, characterize the following: O2 This is a molecule because there is a covalent bond (a double covalent bond in this case). It is not a compound since there is only oxygen, which would make it an element. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Quizicule 1. Draw a possible molecule with the molecular formula C3O3H4 in both lewis-dot (valence shells only), displayed form (all atoms indicated), and skeletal form. (6 points) Chapter 1 3 - Introduction: The MoleculesExploring The of Cells Scientific Life Study of Life How can is are allwelife united? AIM: What the major characteristics of life? AIM: How observe cells? Aside: To what is DNA analagous DNA stores information…books store information…both are pointless without… Chapter 1 3 - Introduction: The MoleculesExploring The of Cells Scientific Life Study of Life How can is are allwelife united? AIM: What the major characteristics of life? AIM: How observe cells? …something to read them. What is this human analogous t Protein…proteins are molecules that do all the work in the cell…they are like people…the workers / little machines…they build the cell, maintain the cell, divide the cell, read the DNA, and much more. Chapter 1 3 - Introduction: The MoleculesExploring The of Cells Scientific Life Study of Life How can is are allwelife united? AIM: What the major characteristics of life? AIM: How observe cells? How large are proteins and DNA relative to the Imagine Madison Square Garden (MSG) is a single cell…how big is a protein in this cell? A protein would be about the size of a tennis ball in MSG and the DNA would be a string with a thickness of 2mm and a length of 1200 miles!!! Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another way to draw molecules/atoms: spacefill Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... Opium poppy fruit that has been cut revealing the fluid containing morphine. In the example to the right, endorphin (a naturally occuring chemical in your brain that reduces the sensation of pain) has its structure mimicked by morphine (a chemical found in opium poppy). This allows morphine to bind the endorphin receptor on the surface of neurons in the brain making it a very popular painkiller. Fig. 2.17 Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... The structure of hemoglobin allows it to carry four oxygen (O2) molecules. Fig. 2.17 Like the structure of a hammer allows it to hits nails… Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Biochemical Pathways…an introduction They are a lot like factory lines: proteins (enzymes) in blue = yellow robots Molecules being modified = cars and parts Equilibrium vs homeostasis Metabolism Basic Research Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Biochemical Pathways…an introduction Mevastatin Professor Akira Endo Basic Research turned Applied Research Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... Lovastatin treats hypercholesterolemia (high cholesterol level). It was first isolated in 1978 from the fungus Asperfillus terreus. Worth a read… Mevastatin Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... Cholesterol Biosynthesis – you don’t need to eat cholesterol. Your enzymes make it. **Enzymes are in blue. **Substrates/Products are in black with final products in GREEN. Lovastatin binds to and inhibits HMG-CoA reductase – remember that enzyme names will usually end in –ase. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... HMG-CoA reductase with lovastatin bound: Lovastatin has a structure that allows it to bind to the active site of this enzyme thereby giving it its function as an inhibitor and in turn reducer of blood cholesterol. Alpha helices are in blue. Beta sheets in purple. Loops in yellow. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: The structure of a molecules determines its function... This is the reaction normally catalyzed by this enzyme. Compare the natural substrate (HMG CoA) to Lovastatin. Could you guess that this molecule would bind to this protein? Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Another structure/function example: Lipitor (Atorvastatin} Born from the understanding of fungal statins… Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Top 20 selling drugs 2014 - Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Quizicule 1. Define Equilibrium. How is it different from homeostasis? 2. What are statins? 3. A) What was the rationale behind the discovery of the first statin? B) In what type of organism was the first statin discovered? 4. What is the difference between basic and applied research? Give an example using statins. 5. Give an analogy for biochemical pathways. Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chemical Reaction The transformation of one set of chemical substances to another… Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chemical Reactions and Chemical Equilibrium Some reactions proceed to completion (irreversible reaction) indicated by the single arrow: CaCO3 + 2HCl → CaCl2 + H2O + CO2 However, many proceed in both directions (reversible reaction) as indicated by the double arrow in the reaction diagram reaching an equilibrium. or Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Define Equilibrium… The rates are the same in both directions… In this case, the rate of the reaction to the right is the same as the rate of the reaction to the left…they are occurring at the same speed essentially… If the sustances are being formed as quickly as they are being used then what can you say about the concentrations of all substances? Concentrations are not changing… Chapter 2 - The Chemical Basis of Life AIM: What’s the matter? Chemical Reactions and Chemical Equilibrium Biological Example: Glycolysis is shown to the right. The circled numbers are enzymes. Which reactions are reversible? Those catalyzed by enzymes 3, 4, 6, 7, 8, 9, 10, and 11 Which reactions are irreversible? Those catalyzed by enzymes 1, 2, 5 and 12