U4Q1.Molecular Geometry (AP)
advertisement
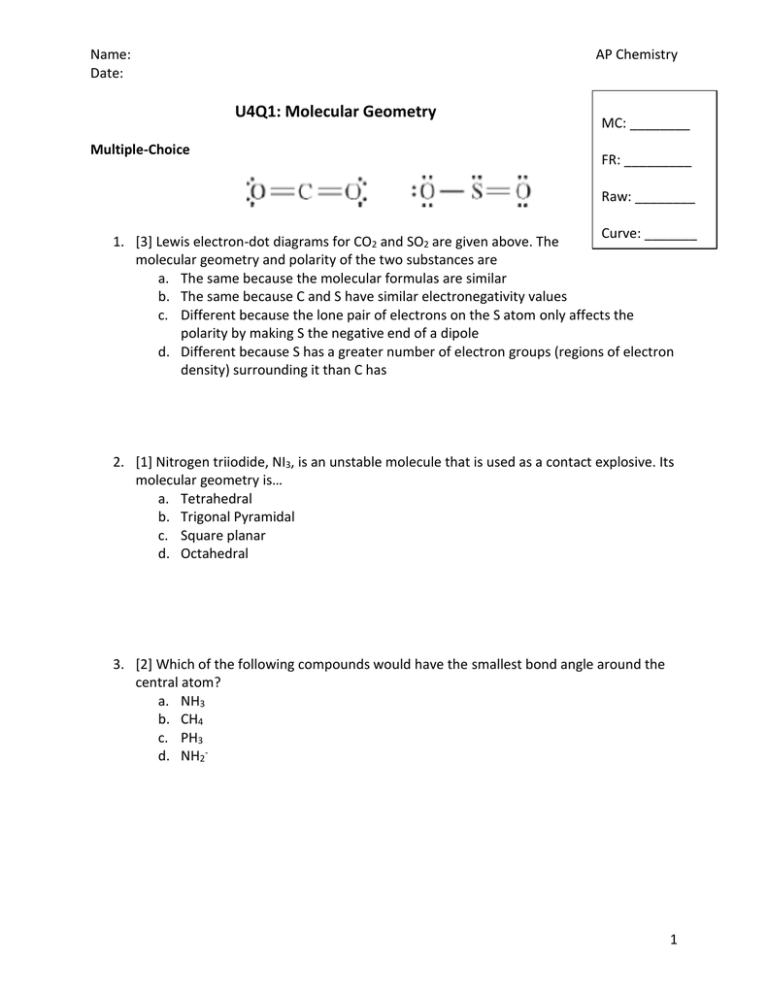
Name: Date: AP Chemistry U4Q1: Molecular Geometry Multiple-Choice MC: ________ FR: _________ Raw: ________ Curve: _______ 1. [3] Lewis electron-dot diagrams for CO2 and SO2 are given above. The molecular geometry and polarity of the two substances are a. The same because the molecular formulas are similar b. The same because C and S have similar electronegativity values c. Different because the lone pair of electrons on the S atom only affects the polarity by making S the negative end of a dipole d. Different because S has a greater number of electron groups (regions of electron density) surrounding it than C has 2. [1] Nitrogen triiodide, NI3, is an unstable molecule that is used as a contact explosive. Its molecular geometry is… a. Tetrahedral b. Trigonal Pyramidal c. Square planar d. Octahedral 3. [2] Which of the following compounds would have the smallest bond angle around the central atom? a. NH3 b. CH4 c. PH3 d. NH2- 1 Name: Date: AP Chemistry 4. [3] Both ammonia (NH3) and methane (CH4) have nearly equivalent bond angles, however only one of these molecules is considered polar. Which statement accurately describes which molecule is polar with the correct reason? a. CH4 polar because it is tetrahedral so all bond angles are equivalent indicating a polar molecule b. NH3 is polar because there is a net dipole moment pointing in the direction of the lone pair c. NH3 is polar because Nitrogen is more electronegative than Carbon d. NH3 is polar because it has a tetrahedral geometry 5. [2] The hybridization of the oxygen atom labeled ‘y’ in the structure below is __________ and bond angle around this atom is __________. a. b. c. d. sp, 180° sp2, 109.5° sp3, 105° sp3, 109.5° 6. [1] Hydrogen Cyanide (HCN) is an extremely poisonous liquid that boils just slightly above room temperature (79 ˚F). How many total sigma bonds would you expect to form in this compound? a. 1 b. 2 c. 3 d. 4 7. [2] Which of the following molecules would have the shortest bond? a. I2 b. CO c. CCl4 d. O2 2 Name: Date: AP Chemistry 8. [2] Based on the Lewis structures drawn for NO3- below, which of the following structures would be the most likely to exist? a. b. c. d. Structure 1 Structure 2 Structure 3 Both Structures 2 and 3 would equivalent 9. [3] The BF3 molecule is nonpolar, whereas the NF3 molecule is polar. Which of the following statements accounts for the difference in polarity of the two molecules? a. In NF3, each F is joined to N with multiple bonds, whereas in BF3, each F is joined to B with single bonds b. N-F bonds are polar whereas B-F bonds are nonpolar c. NF3 is an ionic compound, whereas BF3 is a molecular compound d. Unlike BF3, NF3 has a nonplanar geometry due to an unshared pair of electrons on the N atom 10. [1] What would the bond order be for N2? a. 1 b. 2 c. 3 d. 4 3 Name: Date: AP Chemistry THIS PAGE INTENTIONALLY LEFT BLANK 4 Name: Date: AP Chemistry Free Response 1. Methanamide, CH3NO, is a liquid at 25 ˚C. The complete Lewis structure for methanamide is shown below: a. In the molecule, angle x is not 180˚. Estimate the observed angle. Justify your answer. b. In the molecule, angle y is not 90˚. Estimate the observed angle and explain your answer in terms of electron domains. c. Which bond would you expect to be more polar, the C-H bond or the N-H bond? Explain. d. What would you expect the hybridization to be around each central atom in methanamide? Explain your answer. e. Consider a molecule with the formula CH2O2. The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. In the box below, draw the complete Lewis structure for the molecule. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 5 Name: Date: AP Chemistry ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 6







