Classification of polymerization reactions
advertisement

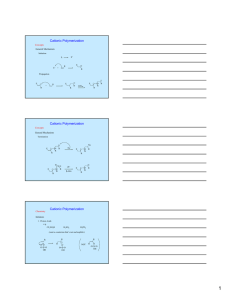
CLASSIFICATION OF POLYMERIZATION REACTIONS For polymerization it is required that the monomer molecule is capable of being linked to two (or more) other molecules of monomer by chemical reaction (functionality). Functionality of two or higher is needed. Classification of polymerization are based on comparison of the molecular formed of a polymer with that of the monomer from which it was formed. CONDENSATION POLYMERIZATION Those which yield polymers with R.U. having less atoms than present in the monomers from which they formed (elimination of a small molecule). Example Polyester is a typical condensation reaction between bifunctional monomers with elimination of water. X HO-R1-OH + X HOCO-R2-COOH HO [ R1-OCO-R2-COO ]x H + (2X-1) H2O ADDITION POLYMERIZATION Those which yield polymers with repeat units having identical molecular formula to those of monomers from which the one formed. Example Polystyrene. X CH2 = CH ----CH2-CH-CH2-CH---, etc. The addition reaction mechanisms are result from chain reactions involving some sort of active center. Because condensation reaction mechanisms are usually formed by the step wise intermolecular condensation of reactive groups. And because some condensation process reacted in addition manner a new term is used to defined this process (step reaction or condensation polymer). See table 2.1 Billmeyer Step Polymerization Linear Step Polymerization Step polymerization involves successive reaction between pairs of maturely-reactive functional groups. Functionality is a critical importance in this reaction Example 1)Condensation reaction of carboxylic acid groups with hydroxyl groups both acetic acid and ethyl alcohol are monofunctional compounds which yield ethyl acetate with elimination of water CH3COOH +CH3CH2OH CH3COOCH2CH3 + H2O From this polymer chain can not form. 2)If we have terephthalic acid and ethylene glycol both are difuctional COOH CH2-OH COOCH2-CH2-OH + COOH + H2O CH2-OH COOH The product is an ester which have one carboxylic acid end group and one hydroxyl end-group (i.e. it is also difunctional). The dimers can react with molecules of acid or alcohol or dimer. formation of difuctional. Difunctional monomers Trifunctional monomers linear step polymerization. branched polymer. Example Tetephtalic acid react with glycerol HO CH2CH (OH) CH2OH The product is non linear. POLYCONDENSATOIN Step polymerization that involve reaction in which small molecules are eliminated are termed polycondensation. Example (1) the formation of linear polyesters n HOOC-R1 - COOH + nHO-R2- OH H [ OOC-R1-COOR2 ]n OH + (2n-1) H2O Where R1 and R2 general groups. It can be represented as R1A2 + R2B2 where: A & B are mutually reactive groups. Polyesters may also prepared from single monomer which contain both types of functional group. Example w-hydroxyl carboxylic acids. HOOC-R-OH This is ARB step polymerization Advantage exact stoiciometric equivalent of the two functional groups is granted. POLYAMIDES Polyamides are prepared by polycondensation of amine groups. Example nH2N-R1-NH2 + nHOOC-R2-COOH H [ NH-R1NHOC-R2-CO ]n OH + (2n-1) H2O POLYETHERS Polyethers formed by dehydration of dioles. HO-R-OH + HO-R-OH (n-1)H2O RA2 step polymerization HO [ RO ]n H + SILOXANE Siloxane Prepared by hydrolysis of dialkylsiloxanes e.g. dichlorodimethylsiloxene. CH3 nCl-Si-Cl + (n+1) H2O CH3 dichloro CH3 HO [ Si-O ] H + 2nHCl CH3 (poly dimethylsiloxane) (PDMS) initially hydrolysis occurs CH3 Cl-Si-Cl + 2H2O CH3 CH3 HO-Si-OH +2HCl CH3 after that we can have both RA2 + RB2 polymerized (CH3)2 SiCl2 react with (CH3)2 Si(OH)2 or (CH3)2 Si(OH2) can condense by itself (RA2) polymer. POLYADDITION Step polymerization in which the monomers react together without the elimination of other molecules. Example 1) Polyurethanes by the RA2 + RB2 reaction of diisocyanate with diols. O = C = N = R1 - N = C = O + HO - R2 - OH O O [ C - NH-R1 - NH-C - OR2-O ]n 2) Polyureas O O C - N - R1 - N = C + H2N - R2-NH2 O O [ C - NH-R1 - NH-C-NH-R2-NH ]n Thank You See You Next Lecture