CHAIN POLYMERIZATION Free radical polymerization
advertisement
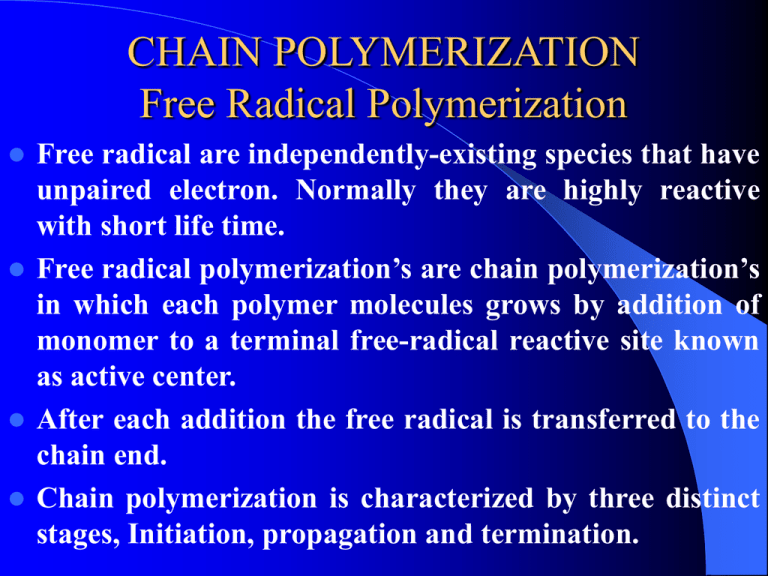
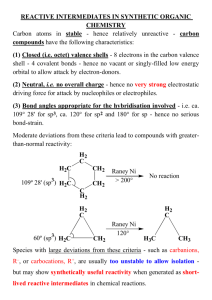
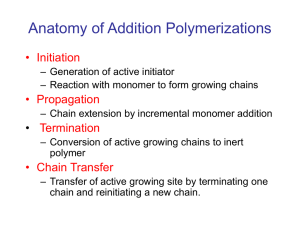
CHAIN POLYMERIZATION Free Radical Polymerization Free radical are independently-existing species that have unpaired electron. Normally they are highly reactive with short life time. Free radical polymerization’s are chain polymerization’s in which each polymer molecules grows by addition of monomer to a terminal free-radical reactive site known as active center. After each addition the free radical is transferred to the chain end. Chain polymerization is characterized by three distinct stages, Initiation, propagation and termination. Example The formation of polyvinyl monomer. CH2 = CHX INITIATION This stage is a two steps stage 1. The formation of free radicals from an initiator. 2. The addition of one of these free radicals to a monomer molecules. Free radical can be formed by two principal 1. Homolytic scission (homolysis) or breakage of a single bond. 2. Single electron transferred to or from an ion or molecule (redox reactions) Homolytic can be achieved by heat (thermolysis) or by light such as U.V. (photolysis). Example O O O -C-0-0-C- 2 Benzoly peroxide Benzolyooxy radicals (CH3)2 C-N = N- C (CH3)2 2(CH3)2C + N2 CN CN Azobisisobutyronitril -C-O CN 2-Cyanopropyl radicals Sometimes the radicals undergo further breakdown (scissions) such as O -C-0 O +C=O (CH3)2 - C-O CH3 CH3 + (CH3)2-C = 0 Methyl acetone radical PHOTOLYSIS Photolysis is the second principle of free radical formation. The advantage of this method is that the formation of free radicals begins at the instant of exposure and cases as soon as the light source is removed. REDOX REACTION Redox reaction defined as the generation of free radicals by electron transfer and it is use when polymerization performed at low temperature. Example CH3 -C-0-0H + Fe2+` CH3 Cumyl Ferrous hydroperoxide ion CH3 -C-O + OH + Fe3+ CH3 Cumyloyloxy radical O O O O-S-O-O-S-O + HO-S-O O O O 0-S-O + O-S-O + OH-S-O O O Sulphate Sulphate Bisulphate ion radical radical O O Presulphate ion Bisulphate ion An active center is crated when a free radical (Ro) which is generated from an initiator attacks the -bond of the monomer molecules. R + CH2 = CH X R-CH2-CH or R-CH - CH2 X X This is more likely This is more stable Sometime free radical react with each other such as: O O O 2 OR 2 -C-0 -C-O- +C=O PROPAGATION The addition of monomer molecules to the active center to grow the polymer chain. There are two modes of chain propagation 1. Head to Tail R-CH2-CH + CH2=CH X X 2. Head to Head R-CH2-CH + CH2=CH X X Again R-CH2-CH-CH2-CH X X R-CH2-CH-CH-CH2 X X mode (1) are more dominant. Therefore polymer structure are like -----CH2-CH-CH2-CH-CH2-CH-CH2-CH-----X X X X Time of addition for each monomer is of the order of a millisecond. Thus several thousands of additions can take place in a few seconds. TERMINATION The last stage of chain reaction in which the growth of the polymer chain terminated (or stopped). There are two mechanisms of termination 1. Combination Coupling together of two growing chains to form a single polymer molecules. polystyrene --CH2-CH + CH - CH2-----CH2-CH- CH-CH2--X X X X 2. Disproportionation when a hydrogen atom move from one growing chain to another H H --CH2-CH + C - C------CH2-CH2 + CH=CH2-- X X H X X Saturated end Unsaturated end group polymer group polymer Thank You See You Next Lecture