Acids and Bases
advertisement
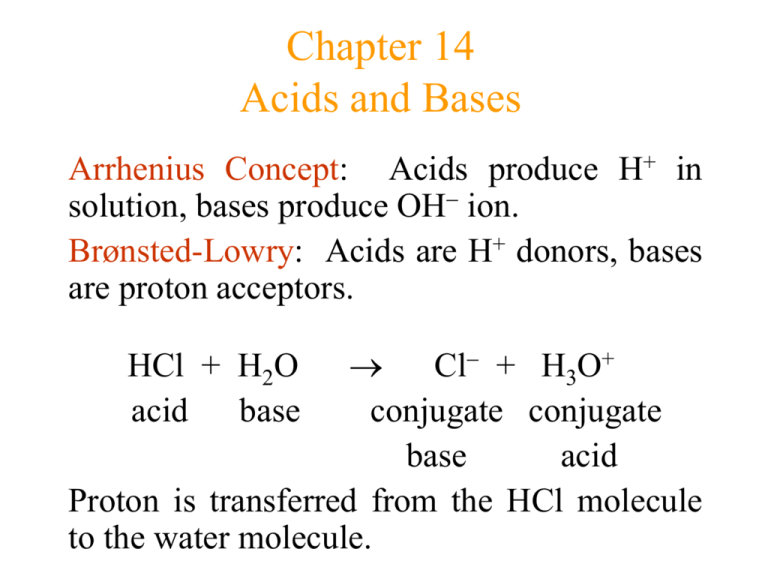
Chapter 14 Acids and Bases Arrhenius Concept: Acids produce H+ in solution, bases produce OH ion. Brønsted-Lowry: Acids are H+ donors, bases are proton acceptors. Cl + H3O+ conjugate conjugate base acid Proton is transferred from the HCl molecule to the water molecule. HCl + H2O acid base Figure 14.1 The Reaction of HCI and H2O Figure 14.2 The Reaction of an Acid with Water Figure 14.3 The Reaction of NH3 with HCI to Form NH4+ and CI- Conjugate Acid/Base Pairs HA(aq) + H2O(l) H3O+(aq) + A(aq) conj acid 1 conj base 2 conj acid 2 conj base 1 Conjugate base: everything that remains of the acid molecule after a proton is lost. Conjugate acid: formed when the proton is transferred to the base. A conjugate acid-base pair consists of two substances related to each other by the donating and accepting of a single proton. Acid Dissociation Constant (Ka) HA(aq) + H2O(l) H3O+(aq) + A(aq) Ka H3O HA A H A HA Where, Ka is the acid dissociation constant. In dilute solution we can assume that the concentration of liquid water remains essentially constant when an acid is dissolved. Acid Strength The strength of an acid is defined by the equilibrium position of its dissociation (ionization) reaction: HA(aq) + H2O(l) H3O+(aq) + A-(aq) Strong Acid: Its equilibrium position lies far to the right. (HNO3) Yields a weak conjugate base. (NO3) Common strong acids are H2SO4, HCl, HNO3, HClO4 Figure 14.4 Graphic Representation of the Behavior of Acids of Different Strengths in Aqueous Solution Figure 14.5 Acid Strength Versus Conjugate Base Strength Acid Strength (continued) Weak Acid: Its equilibrium lies far to the left. (CH3COOH) Yields a much stronger (it is relatively strong) conjugate base than water. (CH3COO) Common weak acids are H3PO4, HNO2, HOCl, organic acids (-COOH). Figure 14.6 A Strong Acid (a) and a Weak Acid (b) in Water Monoprotic acid: One acidic proton (HCl) HCl(aq) H+(aq) + Cl-(aq) Diprotic acid: Two acidic protons (H2SO4) H2SO4(aq) H+(aq) + HSO4-(aq) HSO4- (aq) H+(aq) + SO4 2-(aq) Oxyacids: Acidic proton is attached to an oxygen atom (H2SO4) Organic acids: Those with a carbon atom backbone, contain the carboxyl group (COOH). CH3-COOH, C6H5-COOH Water as an Acid and a Base A substance is said to be amphoteric if it can behave either as an acid or as a base. Water is amphoteric (it can behave either as an acid or a base). H2O + H2O H3O+ + OH conj conj acid 1 base 2 acid 2 base 1 Kw = [H3O+][OH-] = [H+][OH-] = 1 1014 at 25°C Where, Kw is the ion-product constant or dissociation constant for water. [H+] = [OH-] = 1.0 x 10-7 M at 25oC in pure water. Figure 14.7 Two Water Molecules React to Form H3O+ and OH- The pH Scale The pH scale provides a convenient way to represent solution acidity. The pH is a log scale based on 10. pH log[H+] pH in water ranges from 0 to 14. The pH decreases as [H+] increases. Kw = 1.00 1014 = [H+] [OH] pKw = -log Kw = 14.00 = pH + pOH As pH rises, pOH falls (sum = 14.00). pOH = -log [OH-] Figure 14.8 The pH Scale and pH Values of Some Common Substances Calculating the pH of Strong Acid Solutions • Calculate the pH of 1.0 M HCl. Since HCl is a strong acid, the major species in solution are H+, Cl- and H2O To calculate the pH we will focus on major species that can furnish H+. The acid is completely dissociates in water producing H+ and water also furnishes H+ by autoionization by the equilibrium H2O(l) H+(aq) + OH-(aq) In pure water at 25oC, [H+] is 10-7M and in acidic solution even less than that. So the amount of H+ contributed by water is negligible compared with the 1.0M H+ from the dissociation of HCl. pH = -log [H+] = -log (1.0) = 0 Solving Weak Acid Equilibrium Problems List major species in solution. species that can produce H+ and write reactions. Choose Based on K values, decide on dominant equilibrium. Write equilibrium expression for dominant equilibrium. List initial concentrations in dominant equilibrium. Solving Weak Acid Equilibrium Problems (continued) Define Write change at equilibrium (as “x”). equilibrium concentrations in terms of x. Substitute equilibrium concentrations into equilibrium expression. Solve for x the “easy way.” Verify assumptions using 5% rule. Calculate [H+] and pH. Percent Dissociation (Ionization) It is useful to specify the amount of weak acid that has dissociated in achieving equilibrium in an aqueous solution. The percent dissociation is defined as follows: amount dissociated ( M ) % dissociation 100% initial concentration( M ) For a given weak acid, the percent dissociation increases as the acid becomes more dilute. Figure 14.10 The Effect of Dilution on the Percent Dissociation and (H+) of a Weak Acid Solution Bases • Arrhenius concept: A base is a substance that produces OH- ions in aqueous solution. • Bronsted-Lowry concept: A base is a proton acceptor. • “Strong” and “weak” are used in the same sense for bases as for acids. • strong = complete dissociation (hydroxide ion supplied to solution) NaOH(s) Na+(aq) + OH(aq) Bases (continued) weak = very little dissociation (or reaction with water) H3CNH2(aq) + H2O(l) H3CNH3+(aq) + OH(aq) H3CNH2 molecule accepts a proton and thus functions as a base. Water is the acid in this reaction. Methyl amine contains no hydroxide ion, it still increases the concentration of hydroxide ion to yield a basic solution. Polyprotic Acids . . . can furnish more than one proton (H+) to the solution. A polyprotic acid always dissociates in a stepwise manner, one proton at a time. ( Ka1 ) ( Ka 2 ) H 2CO3 H HCO3 HCO3 H CO32 For a typical weak polyprotic acid, Ka1 > Ka2 > Ka3 Acid-Base Properties of Salts Cation neutral neutral Acidic or Basic neutral basic Anion neutral conj base of weak acid conj acid of neutral acidic weak base conj acid of conj base of depends on weak base weak acid Ka & Kb values Example NaCl NaF NH4Cl Al2(SO4)3 Structure and Acid-Base Properties • When a substance is dissolved in water, it produces an acidic solution if it can donate protons and produces a basic solution if it can accept protons. • Two factors for acidity in binary compounds: Bond Polarity (high is good) Bond Strength (low is good) Figure 14.11 The Effect of the Number of Attached Oxygens on the O-H Bond in a Series of of Chlorine Oxyacids Oxides • Acidic Oxides (Acid Anhydrides): When a covalent oxide dissolves in water an acidic solution forms. OX bond is strong and covalent. SO2, NO2, CO2, CrO3 • Basic Oxides (Basic Anhydrides): When an ionic oxide dissolves in water a basic solution results. OX bond is ionic. K2O, CaO Lewis Acids and Bases Lewis Acid: electron pair acceptor Lewis Base: electron pair donor Lewis acid has an empty atomic orbital that it can use to accept an electron pair from a molecule that has a lone pair of electrons. Al3+ + 6 O H Lewis acid H Al H Lewis base 3+ O H 6 Figure 14.13 The AI(H2O)63+ Ion





