CHAPTER 14: ACIDS AND BASES
advertisement

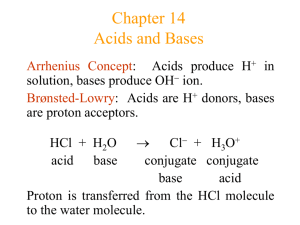
CHAPTER 14: ACIDS AND BASES Definitions of Acid-base: Arrhenius : Acids produce hydrogen ions in aqueous solution, while bases produce hydroxide ions. Bronsted-Lowry : An acid is a proton donor, a base is a proton acceptor. Lewis : An acid is electron pair acceptor, a base is an electron pair donor. General reaction for acid dissociation in water: HA + H2O H3O+ + AAcid Base Conjugate acid Conjugate base Equilibrium expression: Ka = [H3O+][A-] Ka=acid dissociation constant [HA] Conjugate acid : Everything that remains of the acid molecule after a proton is lost. Conjugate base : Formed when the proton is transferred to the base. Conjugate acid-base pair : Two substances related to each other by the donating and accepting of a single proton. Property Strong Acid Weak Acid Large Small Ka value Far to the right Far to the left Position of dissociation [H+] ≈ [HA]o [H+] <<[ HA]o [H+]eq vs. [HA]o Much weaker Much stronger Strength of conjugate base vs. H2O Common strong acids:H2SO4, HCl, HNO3, HClO4, HBr, HI Monoprotic acid: contains one acidic proton, ex. HBr H+ + Br Diprotic acid: contains two acidic protons, ex. H2SO4 (aq) H+ (aq) + HSO4- (aq) HSO4- (aq) H+ (aq) + SO42- (aq) Triprotic acid: contains three acidic protons, ex. H3PO4. Oxyacid: acidic proton attached to an oxygen atom, ex. HOCl. Organic acid: with carbon atom backbone, commonly carboxyl group, ex. C6H5COOH, benzoic acid. For strong acids, Ka can’t be measured accurately because the equilibrium lies so far to the right. Amphoteric: A substance that can act either as an acid or a case, ex. H2O. Autoionization of water: H2O (l) + H2O H3O+ + OHKw = [H3O+][OH-]=1.0e-14 at 25ºC Kw = ion-product constant, always refers to the autoionization of water. Ka x Kb = 1.0e-14 [H3O+] = [OH-] Ka=Kb [H3O+] < [OH-] Kb>Ka [H3O+] > [OH-] Kb<Ka Kw varies with temperature, since it’s an equilibrium constant. The pH Scale The pH scale is a compact way to represent solution acidity. 0-2 :strongly acidic 3-6 :weakly acidic 7 :neutral 8-10 :weakly basic 11-14 :strongly basic/alkaline The number of decimal places for pH, pOH, and pK values equal the number of sig figs for [H3O+],[OH-], and K. pH=-log[H+] pOH=-log[OH-] pH + pOH = 14.00 Calculating the pH of Strong Acid Solutions Strong acids dissociate completely and never reach equilibrium with their conjugate bases. [H+] = [HA]o Calculating the pH of Weak Acid Solutions List the major species in the solution Choose the species that can produce H+, and write balanced equations for the reaction producing H+. Decide the equilibrium that will dominate the production of H+ by looking at Ka values. Write equilibrium expression for the dominant equilibrium List the initial concentrations of the species participating in the dominant equilibrium Define the change needed to achieve equilibrium (x) Write the equilibrium concentrations in terms of x Substitute the equilibrium concentrations into the equilibrium expression Solve for x by assuming [HA]o – x ≈ [HA]o Use the 5% rule to check approximation. Calculate [H+] and pH ice table Percent dissociation= amount dissociated (mol/L) x100% Initial concentration (mol/L) For solutions of weak acid HA, [H+] decreases as [HA]o decreases (more dilute), but the percent dissociation increases as [HA]o decreases. Bases Strong bases dissociate completely. Strong bases: all hydroxides of group 1A elements, and Ca, Sr, Ba. A base doesn’t have to contain hydroxide ions, ex. NH3, CH3NH2, (CH3)2NH, (CH3)3N, C5H5N. General reaction between a base B and water: B + H2O BH+ + OHBase Acid Conjugate acid Conjugate base Equilibrium constant: Kb = [BH+][OH-] [B] pH calculations are very similar to those for acids. Polyprotic Acids are acids that can furnish more than one proton. It dissociates in a stepwise manner, one proton at a time: H2CO3 H+ + HCO3Ka1 HCO3- H+ + CO32Ka2 The conjugate base HCO3- of the first dissociation equilibrium becomes the acid in the second step. For a typical weak polyprotic acid: Ka1>Ka2>Ka3 because as the negative charge on the acid increases, it becomes more difficult to remove the positively charged proton. In pH calculation for polyprotic acid in water, only the first dissociation step is important to determine pH. Sulfuric Acid is unique because the first dissociation is a strong acid and the second is a weak acid: H2SO4 (aq) H+ (aq) + HSO4- (aq) Ka1 is very large HSO4- (aq) H+ (aq) + SO42- (aq) Ka2 = 1.2e-2 Only in dilute (small concentration) H2SO4 does the second dissociation step contribute significantly to [H+]. For large Ka (≥1.0e-2), approximation is invalid (>5%) and quadratic formula must be used. Acid-base property of Salts Type of salt example Comment pH of solution Cation Anion From strong base From strong acid KCl, KNO3 None Neutral from strong base From weak acid NaC2H3O2 Anion = base, Cation= none Basic Conjugate acid of weak base Conjugate acid of weak base From strong acid NH4Cl Cation = acid, Anion = none Acidic Conjugate base of weak acid NH4CN Cation=acid, Anion= base Highly charged metal ion From strong acid FeCl3 Hydrated cation=acid Anion = none Acidic if Ka>Kb Basic if Kb>Ka Neutral if Ka=Kb Acidic Effect of Structure on Acid-Base Properties Factors that determine if a molecule with X-H bold acts as an acid are the strength oh H-X bond and its polarity. H—F>H—Cl>H—Br>H—I least polar. HF bond is very strong, so it’s a weak acid even though it is the most electronegative element. For a given series the acid strength increases with an increase in the number of oxygen atoms attached to the central atom: HClO < HClO2 < HClO3 < HClO4 most polar (mimic ionic bond), electron density is the most on . For hydrated metal ions, the acidity of the water molecules attached to the metal ion is increased by the attraction of electrons to the positive metal ion. The greater the charge on the metal ion, the more acidic the hydrated ion becomes. Al(H2O)63+ > Zn(H2O)42+ Hydrated highly charged metal Generic reaction: or Acid-Base Properties of Oxides Nonmetal Oxide + H2O Acid, ex: SO2 + H2O H2SO4 Metal Oxide + H2O Base, ex: CaO + H2O Ca(OH)2 Questions: 1. Which salt produces the most alkaline solution at a concentration of 0.1 M? a. KNO3 c. NH4Cl b. MgCl2 d. NaNO2 2. Which is not an acid-base conjugate pair? a. HS, S2c. HNO2, NO2e. C6H5COOH, C6H5COOb. H3O+, OHd. CH3NH4+, CH3NH3 3. Which gives a basic solution when dissolved in water? I. NaCH3COO II.NaNO3 III. NH4NO3 a. I only b. II only c. I & II only d. I, II, III e. None of the above 4. The Kb of the weak base, methylamine, CH3NH2 is 4.2e-4. What is the Ka of methylamine? a. 4.2e-18 b. 2.4e-11 c. -4.2e-4 d. 4.2e-4 e. 4.2e-10 5. Which of the following species is amphoteric? a. HNO3 b. HC2H3O2 c. HSO4d. H3PO4 e. ClO4Free response: In water, hydrazoic acid, HN3, is a weak acid that has an equilibrium constant, Ka, equal to 2.8e-5 at 25C. A 0.300 litre sample of a 0.050 molar solution of the acid is prepared. a. Write the expression for the equilibrium constant, Ka, for hydrazoic acid. b. Calculate the pH of this solution at 25C. c. To 0.150 litre of this solution, 0.80 gram of sodium azide, NaN3 is added. The salt dissolved completely. Calculate the pH of the resulting solution at 25C if the volume of the solution remains unchanged. d. To the remaining 0.150 litre of the original solution, 0.075 litre of 0.100 molar NaOH solution is added. Calculate the [OH-] for the resulting solution at 25C. By Diana Ang. Period 4