Molecular Weight - MrAllanScienceGFC
advertisement

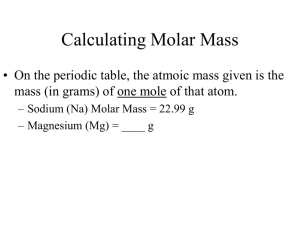
Chapter 10 The Mole Objectives Explain how a mole is used to indirectly count the number of particles of matter Relate the mole to common everyday counting unit Calculate the molar mass of a compound Convert between moles and number of representative particles Convert between number of moles and the mass of an element Convert between number of moles and number of atoms of an element Chemical Quantities Mole – represents a number of items - is the SI base unit used to measure the amount of a substance Just like a dozen = 12, gross = 144, ream =500 Mole has 6.02 x1023 particles of any substance Avogadro’s Constant 6.02 x1023 particles = number of particles in 1 mole of substance We use Avogadro’s number as a grouping Particles can be anything – Cars, people, atoms, molecules How big is a mole? 100 average-sized peas Roughly the volume of a cubic inch 1,000,000 peas (106) Billion (109) peas Fill a refrigerator Fill a house basement to attic Trillion (1012) peas Fill a 1000 houses How big is a Mole? Quadrillion (1015) peas Quintillion (1018) peas Blanket of peas 4 feet deep all over ND Sextillion (1021) peas Fill all the buildings in a larger city like Minneapolis Earth covered in 4 ft Septillion (1024) peas 250,000 planets covered 4ft deep Molecular Weight Molar mass – of any substance is the mass (in grams) of 1 mole of substance 3 different types Molar Masses Each of the following represents 1 mole: 1) Gram Atomic mass (gam) 1 mole of atoms (single element) Can get from periodic table write values to the tenth Examples: Oxygen 1 mole O = 16.0 g Iron 1 mole Fe = 55.9 g Cu+2 1 mole Cu+2 = 63.6 g Molecular Weight 2) Gram molecular mass (gmm) 1 mole of molecules –> used with molecular compounds Add the molar mass of each element multiplied by its subscript Example: What is the molar mass or Gram molecular mass of CO2 ? C O 12.0g x 1 = 16.0g x 2 = 12.0g 32.0g 44.0g 1 mole of CO2 = 44.0g Molecular Weight 3) Gram formula mass (gfm) 1 mole of formula units –>ionic compounds Add the molar mass of each element multiplied by its subscript Examples: What is the molar mass or Gram Formula mass of NaCl ? Na Cl 23.0g x 1 = 23.0g 35.5g x 1 = 35.5g 58.5 g 1 mole of NaCl = 58.5 g Molecular Mass What is the molar mass or Gram Formula mass of Na2SO4 ? Na S O 23.0g x 2 = 46.0g 32.1g x 1 = 32.1g 16.0g x 4 = 64.0g 142.1g 1 mole of Na2SO4 = 142.1g How many molecules of Na2SO4 is there in 1 mole? 6.02 x 1023 molecules of Na2SO4 = 1 mole Na2SO4 Moles to mass conversions 1 mole of substance is equal to its molar mass 1 mole of NaCl = 58.5 g NaCl 2.54 moles of NaCl contains how many grams? 1 ( ( 2.54 moles NaCl ( 58.5 g NaCl 1 mole NaCl ( = 149 g NaCl 252 grams of NaCl is how many moles? ( 1 ( 1 mole NaCl 58.5 grams NaCl ( ( 252 grams NaCl = 4.31 moles NaCl Conversions – Mass/grams/particles 1 ( 252 grams NaCl ( 1 mole NaCl 58.5 grams NaCl ( ( How many particles (Formula Units) of NaCl are there in 252 grams of NaCl ( 6.02 x 1023 FU of NaCl 1 mole NaCl ( = 2.59 x 1024 FU NaCl 1 ( 3.50 x 1024 molecules of NO3 1 mole NO3 ( 6.02 x 10 23 ( ( 3.50 x 1024 molecules of NO3 has a mass of how many grams? molecules of NO3 = ( 62.0 grams NO3 1 mole NO3 360. grams NO3 ( Flow chart for conversions Mass of Compound Number of Moles of compound Molar Mass Avagadro’s Number Number of particles Atoms Molecules Formula Units Volume of a Mole of a Gas Volume of 1 mole of gas varies with Δ in temperature Δ in pressure Gases usually measured @ Standard temp and pressure (STP) 0° and 101.3 KPa or 1 ATM 1 Mole of any gas occupies a volume of 22.4L 22.4 L Know as Molar Volume of Gas Mole to a gas Example: 25 g of Neon = ? L 20.2 g Ne ( ( 1 ( 1 mole Ne ( 22. 4 L Ne 1 mole Ne ( ( 25 g Ne = 28 L Ne Percent Composition of a Compound Percent composition – amounts, by % mass, of each element in a compound 2 types of problems 1) Percent composition – relative amounts you are provided amounts % mass of element = grams of element x 100 grams of compound Example: Find the percent composition of the compound that is formed from 128.0 g of sulfur and 192.0 g of oxygen. Example: Find the percent composition of the compound that is formed from 128.0 g of sulfur and 192.0 g of oxygen. 192.0 g +128.0 g 320.0 g Total mass of compound % mass of S = 128.0 g x 100 = 40.00 % S 320.0 g % mass of O = 192.0 g x 100 = 320.0 g 60.00 % O Percent Composition of a Compound 2) Percent composition of known compound You are given the composition Step 1 – use the chemical formula to find molar mass Step 2 – for each element Find the mass %mass Divide it grams of element by molar mass of compound Multiply by 100 = mass of 1 mole of element x 100 molar mass of compound Example Find the percent composition of Cu, SO4 and H2O in CuSO42H2O Empirical Formula Empirical Formula – lowest whole-number ratio of elements in a compound Example C3 H 6 O C6H12O2 Empirical formula Molecular formula The percentage composition of diborane is 78.1% B and 21.9% H Determine the empirical formula. Empirical Formula Step 1 Change % to grams 78.1% B = 78.1 g B 21.9% H = 21.9 g H Step 2 Change the grams to moles for each element (78.1 g B ) (1 mole B ) = 7.23 mole B 1 (10.8 g B) (21.9 g H) (1 mole H ) = 22 mole H 1 (1.0 g H ) Empirical Formula Step 3 Divide each amount by the smallest amount of moles Use these Ratios to determine the Empirical Formula 7.23 moles = 1.00 B 7.23 moles BH3 22 moles = 3.0 H 7.23 moles