The Mole
advertisement
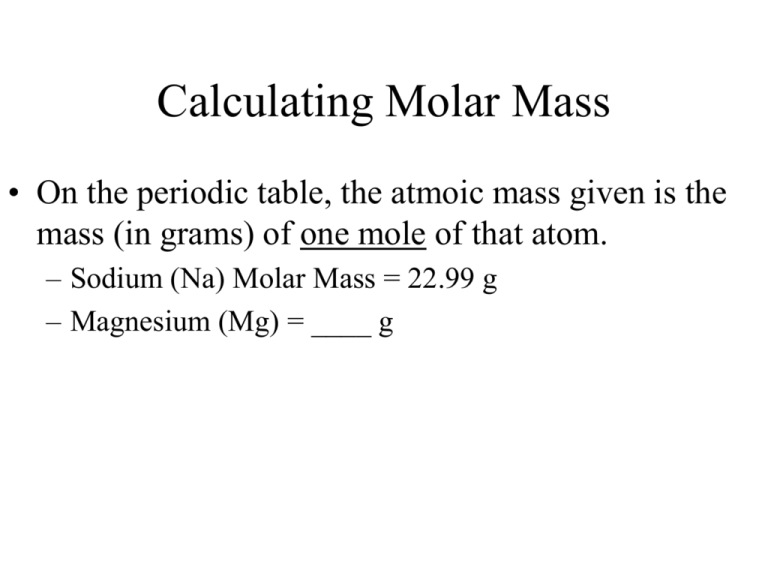
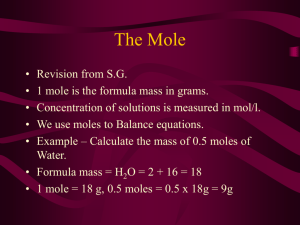
Calculating Molar Mass • On the periodic table, the atmoic mass given is the mass (in grams) of one mole of that atom. – Sodium (Na) Molar Mass = 22.99 g – Magnesium (Mg) = ____ g What about molecules? • NaCl • has one of each atom • = 23 + 35.5 = 58.5 g in one mole of NaCl • H20 • has 2 hydrogen and 1 oxygen • = 2 (1) + 16 = 18 g in one mole of H20 • You need to be very careful when counting the number of atoms of each element in a molecule. • Count the number of atoms for each element in the molecule: • Ca(OH)2 • Na2(CO3) • CH3OH Find the molar mass • • • • • NH3 CO3 HCl Mg(OH)2 Fe3(PO4)2 Converting moles to mass • Determine the molar mass of one mole of that molecule • Use a conversion factor • 1 mole = ____ g (of that molecule or atom) Determine the number of moles: • 25 g Li • 251 g F2 • 345 g 5(H20) Determine the mass: • 1.25 moles of Na • 2.63 moles of CuSO4 • 0.73 moles of Mg(OH)2 Volume • 1 mole = 22.4 L • How many grams are there in 28.6 L of carbon monoxide?