Lewis Structure
advertisement
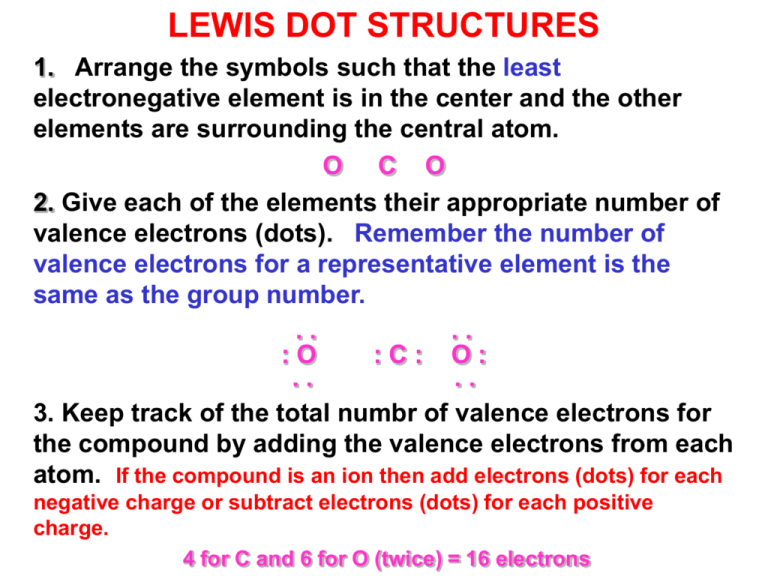
LEWIS DOT STRUCTURES 1. Arrange the symbols such that the least electronegative element is in the center and the other elements are surrounding the central atom. O C O 2. Give each of the elements their appropriate number of valence electrons (dots). Remember the number of valence electrons for a representative element is the same as the group number. .. .. :O :C: O: .. .. 3. Keep track of the total numbr of valence electrons for the compound by adding the valence electrons from each atom. If the compound is an ion then add electrons (dots) for each negative charge or subtract electrons (dots) for each positive charge. 4 for C and 6 for O (twice) = 16 electrons LEWIS DOT STRUCTURES 4. Now move the dots around so that you have 8 dots (the octet rule) around each element (do not forget the exceptions) while at the same time keeping the dots in pairs. Electrons, at this point, exist as pairs (the buddy system). 5. EXCEPTIONS TO THE OCTET RULE: Group I, II, and III need only 2, 4, and 6 electrons, respectively, around that atom. 6. If there are too few pairs to give each atom eight electrons, change the single bonds between two atoms to either double or triple bonds by moving the unbonded pairs of electrons next to a bonding pair. .. .. :O: :C: : O: LEWIS DOT STRUCTURES 7. Once the octet rule has been satisfied for each atom in the molecule then you may replace each pair of dots between two atoms with a dash. .. .. :O =C= O: 8. Now check your structure by a) count the total number of electrons to make sure you did not lose or gain electrons during the process. b) Use FORMAL CHARGE (FC) calculations as a guideline to the correct structure. A zero formal charge is usually a good indication of a stable structure. FC (X) = # of valence electrons - (1/2 bonding electrons + nonbonding electrons) For our example: FC(C) = 4 - (1/2 8 + 0) = 0 FC(O) = 6 - (1/2 4 + 4) = 0 The Basics: Drawing Lewis Structures Step 1: Calculate the total number of valence electrons in the molecule or ion Step 2: Determine the central atom(s) of the molecule or ion – usually it’s the least electronegative atom. Step 3: Draw a tentative diagram for the molecule or ion. Rules a) A hydrogen atom always forms one bond. Hydrogen is always a terminal atom in a Lewis diagram – an atom that is bonded to only one other atom. b) A carbon atom normally forms four bonds c) When several carbon atoms appear in the same molecule, the are often bonded to each other. (No cyclic compounds in Chem 60/68) Draw the Lewis Structure for the following molecules. 1. H2O Oxygen has 6 valence electrons & Hydrogen has 1 valence electron for a total of 8 electrons. .. H:O:H .. 2. CO Oxygen has 6 valence electrons & Carbon has 4 valence electrons for a total of 10 electrons. :C:::O: Draw the Lewis Structure for the following molecules. 3. BH3 Boron has 3 valence electrons & Hydrogen has 1 valence electron for a total of 6 electrons H:B:H .. H 4. NH3 Nitrogen has 5 valence electrons & Hydrogen has 1 valence electron for a total of 8 electrons .. H:N:H .. H p. 362 p. 374 p. 362 p. 375 Cations p. 363 Anions p. 366 Resonance p. 366 LEWIS DOT STRUCTURES Predict the most stable structure: ONC- or OCN- or NOC.. .. .. .. .. .. :O::N::C: or :O::C::N: or :N::O::C: 1) Total electrons is: 6 e- for O + 5 e- for N + 4 e- for C + 1 e- for negative charge = 16 etotal. All structures fulfill the octet rule. 2) FC (X) = # of valence electrons - (1/2 bonding electrons + nonbonding electrons) structure#1: FC(C) = 4 - (1/2 4 + 4) = -2 FC(O) = 6 - (1/2 4 + 4) = 0 FC(N) = 5 -(1/2 8 + 0) = +1 structure#2: FC(C) = 4 - (1/2 8 + 0) = 0 FC(O) = 6 - (1/2 4 + 4) = 0 FC(N) = 5 -(1/2 4 + 4) = -1 structure #3: FC(C) = 4 - (1/2 4 + 4) = -2 FC(O) = 6 - (1/2 8 + 0) = +2 FC(N) = 5 -(1/2 4 + 4) = -1 structure #2 has the combination with the lowest formal charge. It also has the negative formal charge on one of the more electronegative atoms. Calculate the formal charge for the most stable structure: .. :O:C:::N: .. (-1, 0, 0) Practice Problem #13 ClO2.. .. .. : O : Cl : O : .. .. .. SiH4 H ¨ H : Si : H ¨ H AsH3 .. H : As : H ¨ H Group Study Problem #13 1. Draw the Lewis structure for the following a) H2S b) PH3 c) CH2O d) NO2- e) H2CO3 f) CBr4 g) CH2FCl h) C2H2 I) O3 2. Calculate the formal charge for “c”, “d”, “f”, and “I”.