AP Notes Chapter 18
COMMON ION
EFFECT
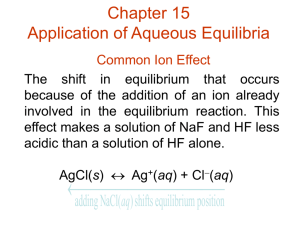
COMMON ION
an ion common with one in a system at equilibrium which places a stress on the equilibrium
Common Ion
Uses of Common
Ion Effect
1. control pH of a weak acid or base
2. control formation of a precipitate
BUFFER
Example
Non-example
A solution which resists a change in pH when an acid or base is added consists of a weak acid or base and a salt containing a common ion of its conjugate
How does
LeChatelier’s Principle explain the operation of a buffer?
Example of a buffer system
CH
3
COOH + HOH
CH
3
COO + H
3
O +
NaCH
3
COO
(aq)
Na + + CH
3
COO -
Characteristics of a
Good Buffer
1. operates over a narrow pH range (< 1 pH unit)
2. no reactions between buffers in a multiple buffer system
3. range can be extended using more than one buffer
pH
pK a
log
[ A
]
[ HA ]
Henderson-Hasselbalch
Equation
pH
pK a
log
[ A
]
[ HA ]
Maximum buffering will occur when ratio is close to 1, or when pH = pK a
1. What is the pH of a
0.20 M acetic acid solution?
Add 10.0 mL of 0.20 M
NaOH to 50.0 mL of the preceding solution.
What is the pH?
Add 5.0g sodium acetate (MM 82.05) to
500. mL of the 0.20 M acetic acid solution.
What is the pH?
Add 10.0 mL of 0.20 M
NaOH to 50.0 mL of the preceding solution.
What is the pH?
2. Calculate the mass of ammonium chloride
(MM 43.6) needed to buffer 250. mL of 2.0 M ammonia to a pH of 10.
TITRATION
CURVES
Titration Curve
A graphical history of a titration typically a plot of the pH
(dependent variable) and volume titrant
(independent variable)
Uses of Titration Curves
1. determine equivalence point
2. determine number of ionization reactions
3. determine optimum buffer region
4. determine possible indicators
Shape of Titration Curve
Strong acid - strong base
Weak acid - strong base
14
12
10
8
6
4
2
0
0
Shape of Titration Curve
5 10 15 20 25 30
Volume NaOH (mL)
HAc-NaOH
35 40 45 50
14
12
10
8
6
4
2
0
0
Shape of Titration Curve
5 10 15 20 25
Volume NaOH (mL)
30
HCl-NaOH
35 40 45 50
Equivalence Point
1. Midpoint between points of inflection
2. Plot of the slope of each point of the curve against volume titrant
(
D pH/
D
V vs V avg
)
Number of
Ionization Reactions
CH
3
COOH - NaOH
H
2
C
2
O
4
- NaOH
Titration Curve of Acetic Acid
14.00
12.00
10.00
8.00
6.00
4.00
2.00
0.00
0 5 10 15 20 25
Volume NaOH (mL)
30 35 40
Titration Curve of Phosphoric Acid
14.00
12.00
10.00
8.00
6.00
4.00
2.00
0.00
0 5 10 15 20 25 30
Volume NaOH (mL)
35 40 45 50
Optimum Buffer Region
Area where the concentration of molecules and their conjugate ions are relatively high
Indicators
Need to choose for each titration system
Dependent on pH at equivalence point
ACID-BASE
INDICATORS
Acid-base indicators are weak Bronsted-
Lowry compounds that are different colors in acid and base form.
Acid-base indicators are all large organic molecules.
HIn <===> H + + In -
Color 1 Color 2
HO
OH
C
OH
C O
-
O
Phenolphthalein
Colorless acid form, HIn
-
O
C
C O
-
O
Phenolphthalein
Pink base form, In -
O
The color change occurs at a different pH for different indicators.
The pH at which the indicator changes color is dependent on the K a of the indicator as a weak acid.
HIn <===> H + + In -
K a
[ H
][ In
]
[ HIn ] or pH
pK a
log
[ In
]
[ HIn ]
Experiments have shown that the minimum amount of change of
HIn <==> In that can be detected visually is
[ In
]
[ HIn ]
1
10 or
10
1
pH
pK a
log
[ In
]
[ HIn ]
but if
In
[
[
HIn
]
] pH
pK a
1
10 or
10
1
, then
1 or pK a
pH
1
Thus, from the
Henderson-Hasselbalch equation, one can select an appropriate indicator for a titration based upon the K a of the indicator and the pH at the equivalence point.
What is the pH at the equivalence point of a titration of 25.0 mL each of 0.10 M HCl and 0.10 M NaOH?
What is the pH at the equivalence point of a titration of 25.0 mL each of 0.10 M
CH
3
COOH and 0.10 M
NaOH?
Phenolphthalein
K
a
= 1 x 10
-9
pH of perceptible color change?
SOLUBILITY
EQUILIBRIA
Saturated Solution
Maximum amount of solute dissolved in a specific volume of solvent at a specific temperature
Saturated Solution
Equilibrium is established between a solid solute and ions from the solute
Super-Saturated Solution
More than the normal maximum amount of solute is dissolved in a solution.
Question
at a constant temperature, what is the difference in concentration of a saturated solution:
(1 mL vs 1 ML solution)
(1 mg vs 1 kg solid)
The concentration of a saturated solution remains the same, no matter how much solid is present, as long as the temperature remains constant.
The “concentration” of a solid remains the same at a constant temperature.
By convention, equations for the formation of saturated solutions are written in the format solid <===> solution
AgCl(s) <===> Ag + + Cl -
AgCl(s) <===> Ag + + Cl -
K eq
[ Ag
][ Cl
]
[ AgCl ]
AgCl(s) <===> Ag + + Cl -
K eq
[ Ag
][ Cl
]
[ AgCl ] but , [ AgCl ] is a constant,
K eq
[ AgCl ]
[ Ag
][ Cl
]
K sp
[ Ag
][ Cl
]
3. What is the solubility of silver chloride in water at 25 o C?
(K sp
= 1.6 x 10 -10 )
4. What is the solubility of lead(II) bromide at
25 o C? (K sp
= 4.6 x 10 -6 )
6. What mass of nickel is dissolved in 100. mL of saturated nickel(II)
(K hydroxide? sp
= 1.6 x 10 -16 )
What is the pH of this solution?
Which is more soluble?
Ag
2
CO
3
CaCO
3
[K sp
= 8.5 x 10 or
-13 ]
[K sp
= 3.4 x 10 -9 ]
SOLUBILITY
----
ACIDITY
----
PRECIPITATION
8. If 0.581 gram of magnesium hydroxide
(MM 58.3) is added to
1.00L of water, will it all
(K sp dissolve?
= 8.9 x 10 -12 )
Below what pH would the solution be buffered so that it does all dissolve?
9. Calculate the concentration of NH
4
+ ammonium chloride from required to prevent the precipitation of Ca(OH)
2 liter of solution that in a contains 0.10 mole of ammonia and 0.10 mole of calcium ion.
10. If 50. mL of 0.012M barium chloride are mixed with 25 mL of 1.0 x 10 -6 M sulfuric acid, will a precipitate form?
HINT: use the concentration quotient “Q” as we used it before
11.You have a aqueous solution of Zn 2+ and Pb 2+ both 0.0010 M. Both form insoluble sulfides.
Approximately what pH will allow maximum precipitation of one ion and leave the other in solution?
[K sp
[K sp
ZnS = 2.5 x 10 -22 ]
PbS = 7 x 10 -29 ]
SOLUBILITY
----
COMMON IONS
----
COMPLEX IONS
12. Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water and in 0.010M
NaSCN.
Complex Ion
A charged species consisting of a metal ion surrounded by ligands
LIGAND
An ion or molecule, acting as a Lewis base, attached to the central metal ion using the d-orbitals of the metal
Coordination Number
The number of ligands attached to the central metal ion.
2, 4, or 6 are most common CN
Metal ions add ligands one
Ag + + NH
3 step at a time.
<==> Ag(NH
3
) +
Ag(NH
3
) + + NH
3
K f1
= 2.1 x 10 3
<==> Ag(NH
3
)
2
+
K f2
= 8.2 x 10 3 where K f
= formation constant
Ag
K f
2NH
3
Ag
[
[ Ag ( NH
3
Ag
][
)
NH
2
3
] 2
]
(
NH
3
K f 1
)
2
K f 2
You need to familiarize yourself with “typical” complex ions, Appendix K
Note that a formation constant reflects the stability of the complex.
13. Calculate the equilibrium constant for
AgI(s) + 2NH
3
(aq) <===>
[Ag(NH
3
)
2
] + (aq) + I (aq)
14. Will 5.0 mL of 2.5 M
NH
3 dissolve 0.0001 mole
AgCl?
15. A solution is prepared by adding 0.10 mole
Ni(NH
3
)
6
Cl
2 to 0.50 L of 3.0
M NH
3
. Calculate the
[Ni(NH
3
)
6
2+ ] and [Ni 2+ ] in the solution.