Notes 22 - CEProfs
advertisement

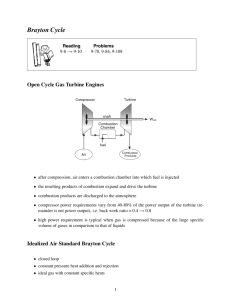
Lec 22: Diesel cycle, Brayton cycle • For next time: – Read: § 8-8 to 8-10. – Methods section due Monday, November 17, 2003 • Outline: – Diesel cycle – Brayton cycle – Example problem • Important points: – Understand differences between the Otto, Diesel and Brayton cycles – Know how to compute the cycles using variable or constant specific heats – Know what components make up each of the cycles. Brayton Cycle Proposed by George Brayton in 1870! http://www.pwc.ca/en_markets/demonstration.html 3 Other applications of Brayton cycle • Power generation - use gas turbines to generate electricity…very efficient • Marine applications in large ships • Automobile racing - late 1960s Indy 500 STP sponsored cars Schematic of simple cycle TEAMPLAY • What expression of the first law applies? u or h? Why? 6 Idealized Brayton Cycle Brayton Cycle 8 Brayton Cycle • 1 to 2--isentropic compression • 2 to 3--constant pressure heat addition (replaces combustion process) • 3 to 4--isentropic expansion in the turbine • 4 to 1--constant pressure heat rejection to return air to original state 9 Brayton Cycle • Because the Brayton cycle operates between two constant pressure lines, or isobars, the pressure ratio is important. • The pressure ratio is not a compression ratio. 10 Brayton cycle analysis As with any cycle, we’re going to concern ourselves with the efficiency and net work output: Efficiency: Net work: w net q in w net w turb w comp Brayton cycle analysis 1 to 2 (isentropic compression in compressor), apply first law: wcomp h 2 h 1 When analyzing the cycle, we know that the compressor work is in (negative). It is standard convention to just drop the negative sign and deal with it later: w comp h 2 h1 12 Brayton cycle analysis 2 to 3 (constant pressure heat addition treated as a heat exchanger) q in q 23 h 3 h 2 13 Brayton cycle analysis 3 to 4 (isentropic expansion in turbine) w turb h 4 h 3 , or w turb h 3 h 4 14 Brayton cycle analysis 4 to 1 (constant pressure heat rejection) q out h1 h 4 , We know this is heat transfer out of the system and therefore negative. In book, they’ll give it a positive sign and then subtract it when necessary. q out h 4 h1 15 Brayton cycle analysis Let’s get the net work: w net w turb w comp Substituting: w net (h 3 h 4 ) (h 2 h1 ) Brayton cycle analysis Let’s get the efficiency: w net (h 3 h 4 ) (h 2 h1 ) q in (h 3 h 2 ) (h 4 h1 ) 1 (h 3 h 2 ) Brayton cycle analysis Let’s assume cold air conditions and manipulate the efficiency expression: 1 c p (T4 T1 ) c p (T3 T2 ) T1 T4 T1 1 1 T2 T3 T2 1 Brayton cycle analysis Using the isentropic relationships, T2 p 2 T1 p1 k 1 k ; T4 p4 T3 p3 k 1 k p1 p2 k 1 k Let’s define: P2 P3 rp pressure ratio P1 P4 19 Brayton cycle analysis Then we can relate the temperature ratios to the pressure ratio: T3 T2 k 1 k rp T1 T4 Plug back into the efficiency expression and simplify: 1 1 k 1 k rp Brayton cycle analysis 21 What does this expression assume? 1 1 k 1 k rp Brayton cycle analysis An important quantity for Brayton cycles is the Back Work Ratio (BWR). BWR w comp w turb Why might this be important? The Back-Work Ratio is the Fraction of Turbine Work Used to Drive the Compressor EXAMPLE PROBLEM The pressure ratio of an air standard Brayton cycle is 4.5 and the inlet conditions to the compressor are 100 kPa and 27C. The turbine is limited to a temperature of 827C and mass flow is 5 kg/s. Determine a) the thermal efficiency b) the net power output in kW c) the BWR Assume constant specific heats. Draw diagram P 2 3 1 4 v Start analysis Let’s get the efficiency: 1 1 k 1 k rp From problem statement, we know rp = 4.5 1 1 1.41 1.4 4.5 0.349 Net power output: Net Power: m w net m w turb w comp W net Substituting for work terms: m (h 3 h 4 ) (h 2 h1 ) W net Applying constant specific heats: m cp (T3 T4 ) (T2 T1 ) W net Need to get T2 and T4 Use isentropic relationships: T2 p 2 T1 p1 k 1 k T4 p 4 ; T3 p 3 k 1 k T1 and T3 are known along with the pressure ratios: Solving for temperatures: T2: T2 3004.5 T4: T4 11000.222 461 K 0.4 1.4 0.4 1.4 715.7 K Net power is then: Wnet (5 kg/ s)(1.0035 kJ / (kg K )) (1100 715.7) (461 300) 1120 kW W net K Back Work Ratio BWR w comp w turb h 2 h1 h3 h4 Applying constant specific heats: T2 T1 461 300 BWR 0.42 T3 T4 1100 715.7 Brayton Cycle We can also do the analysis with variable specific heats….we’ll use relative pressures. p r2 p2 p2 p1 p r1 p1 s p r3 p 2 p3 p3 p 4 p 4 s p r4 p1 32 TEAMPLAY Work the same problem with EES using variable specific heats. How do the numbers change? Brayton Cycle • In theory, as the pressure ratio goes up, the efficiency rises. The limiting factor is frequently the turbine inlet temperature. • The turbine inlet temp is restricted to about 1,700 K or 2,600 F. • Consider a fixed turbine inlet temp., T3 For fixed values of Tmin and Tmax, the net work of the Brayton cycle first increases with the pressure ratio, then reaches a maximum at rp=(Tmax/Tmin)k/[2(k-1)], and finally decreases Brayton Cycle • Irreversibilities • We had wc = h2 – h1 for the ideal cycle, which was isentropic • And we had wt = h3 – h4 for the ideal isentropic cycle Brayton Cycle • In order to deal with irreversibilities, we need to write the values of h2 and h4 as h2,s and h4,s. • Then w t ,a h 3 h 4,act t w t ,s h 3 h 4,s w c ,s h1 h 2,s c w c ,a h1 h 2,act Effect of irreversibilities on the cycle TEAMPLAY Problem 8-69