Cell biology Lab.6
advertisement

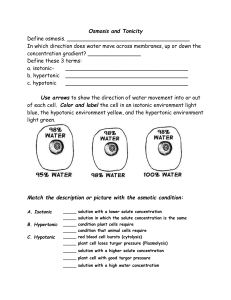
Osmosis Osmosis The diffusion of water across a differentially permeable membrane has been given a special name it is called, Osmosis , Osmotic pressure develop on all side of the membrane that has the higher solute concentration. Tonicity Tonicity refers to the strength of a solution in relationship to Osmosis, cells can be placed in solution that have the same percentage of solute (isotonic solution), a higher percentage of solute (hypertonic-solution),or a lower percentage of solute (hypotonic solution ),that the cell. -In the laboratory, cells are normally placed in solution that causes them neither to gain nor to lose water. Such a solution is said to be an isotonic solution, that is, the solute concentration is the same on both sides of the membrane, and therefore there is no net gain or loss of water. The prefix iso means the same as and the term tonicity refers to the strength of the solution . A 0.9% solution of the salt sodium chloride NaCl is known to be isotonic to red blood cells because the cell neither swell nor shrink when placed in this solution. -Any concentration of salt solution lower than 0.9% is hypotonic to red blood cells. The prefix hypo means less than Red blood cell placed in such a solution expand and sometimes burst due to the buildup of pressure caused by the inward movement of water. The term lysis is used to refer to disrupted cells, heamolysis, then process of red blood cells. Solutions that cause cells to shrink or to shrivel due to a loose of water are said to be hypertonic solution. The prefix hyper means more than and refers to a solution with a higher percentage of solute (less-water) than the cell. A 10% solution of Nacl is hypertonic to red blood cells. If red blood cells placed in this solution, they shrink. The term crenation refers to red blood cells in this condition. Isotonic Hypotonic A-No net movement of water cell water enters the cell Hypertonic water exits the cell Procedure After preparing the 3 solutions ; hypertonic , isotonic and hypotonic solution , then we mix the blood with each one of these solutions in a tube and left stand for 30 - 45 minutes .After that the blood is examined for these changes by making blood smear :By putting a small drop of blood on the slide and by using another clean slide as a “spreader”, touch the small drop with the spreader and allow the blood to run along its edge. away from the largest drops, keeping the spreader at an angle of 45°. Allows the smear to dry in a flat level position protected from flies, dust, external heat. Put an oil and examine under oil immersion . Hypertonic solution : the blood cells will shrink.( crenation) Isotonic solution : the blood cells will not change. Hypotonic solution : the blood cells will swell , burst and lysis.