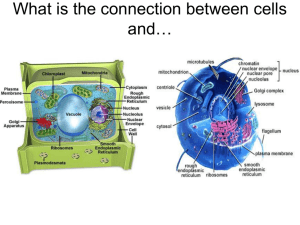
Document
Do you want me to diffuse?
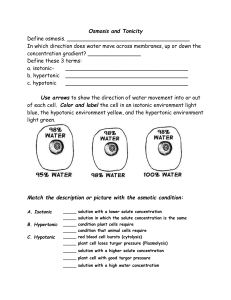
TONICITY-
REVIEW FROM LAST CLASS, PG 74-75
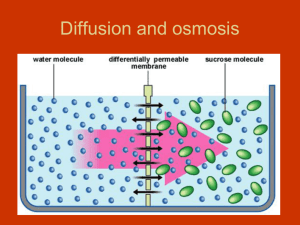
• Tonicity refers to the strength of solution in relationship to osmosis.
• 3 terms to know:
1.
Isotonic
2.
Hypotonic
3.
Hypertonic
The strength of solution will determine the direction water will move!
ISOTONIC SOLUTIONS
• iso = “the same” & tonic = “solution”
• concentration of solute outside cell is the same as the solute concentration in the cell
• water moves into cell at equal rate to water moving out
• cell stays the same, is at EQUILIBRIUM
HYPOTONIC SOLUTIONS
• hypo = “lower”
• solute concentration OUTSIDE cell = LOWER
• More water outside cell water moves INTO cell
• cell swells
• LYSIS –bursting may occur in animal cells
• TURGOR PRESSURE builds up in plant cells
Vacuole pushes outwards against cell wall
HYPERTONIC SOLUTIONS
• hyper= “ higher”
• solute concentration outside cell is higher
• water concentration is lower outside cell
• net movement of water OUT OF cell
• CRENATION occurs in animal cells
• PLASMOLYSIS occurs in plant cells
A) B) C)
D) E) F)
HOW MIGHT THESE SITUATIONS RELATE?
How can we get this ring off?
Can tonicity
Help?
YOUR MISSION TODAY:
• Work on the critical thinking Qs using the tonicity vocab to EXPLAIN…
• Present your Cell Membrane Model to me
• Prepare for CHECKPOINT NEXT CLASS on:
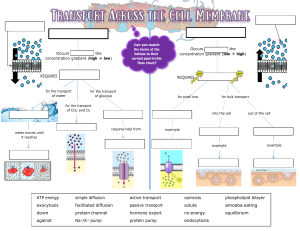
Cell Membrane, Diffusion, Osmosis
OSMOSIS LINKS
• http://www.tvdsb.on.ca/westmin/science/sbi3a1/Cells/Osmosis.htm
(tonicity)
• http://ull.chemistry.uakron.edu/genobc/animations/collapse.mov
(crenation)