The Failure of Skin Substitutes
advertisement

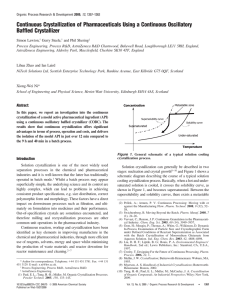
The Crystallizer Simone Houng April 2nd, 2004. Where We Are 191 kg insulin input from ultrafilter Recovery from acetonitrile using zinc chloride 188 kg insulin crystal out of crystallizer to basket centrifuge 98% recovery of insulin crystals Crystallization Formation of solid crystals from a solution Important S-L separation technique Goals: Isolate insulin from the product streams Remove impurities – – Acetonitrile (RP-HPLC) Host cell proteins, trypsin enzyme, byproducts of the transpeptidation reaction, insulin ester Nucleation 1o nucleation – 1st crystals in unseeded matrix • Can be modeled = rate of nucleation 2o nucleation- growth, dominant in bulk crystallization • Much more complicated process Crystal Growth Rate Affect: Morphology (physical characteristics) May determine future product handling Is affected by: Solvent and impurities - large effect Supersaturation Imperfections in crystal lattice CSD (Crystal Size Distribution) Determines processing and product procedures – – – – Size distribution Morphology Polymorphism Impurities in crystal lattice What we Need Define supersaturation- size and properties of product Vessel with sufficient residence time for crystal growth Mixing to ensure uniform crystal growth Difficulties in Scaling Up Need to assume well-mixed and wellsuspended crystals Quality is sensitive to size of reactor Difficult to model because fluid dynamics at different areas affect kinetics crystal quality For batch processes, modeling is often too complex and experimental data is used instead Most Common Methods 1. Cooling- heat sink 2. Solvent evaporation – [solute] 3. Drowning- add non-solvent to solute solubility 4. Chemical reaction- may solubility of solid Alternative Crystallizers Dominant types: Tank Crystallizers Forced Circulation (FC) Fluidized Bed Draft Tube Baffles(DTB) Tank Crystallization Simple stirred batch reactor Advantages: – – For pharmaceuticals, where uniform, well-defined crystals are important High value, low volume products Disadvantage: – – Labor is costly Longer time Forced Circulation (FC) For evaporation & cooling Advantage: Can easily control circulation rates and velocities – Disadvantages: – – – http://www.setprocess.com/technology/fcc.html High heat No stirrer large range of concentrations and temperatures Full cross-section of vessel is not used for crystallization Fluidized Bed Advantages: – Large, uniform size http://scholarsportal.info/pdflinks/04030101195012367.pdf Disadvantages: – Low production rate compared to FC • • – velocity restricted by fluidized requirements Supersaturation of liquid must be low Low birth rate of new crystals Draft Tube Baffles (DTB) Propeller inside fixed tube Preferential fines removal and classified product Little crushing of crystals Uniform concentration with little dead space Large crystals http://www.tsk-g.co.jp/en/tech/uni/uni1. Choosing a Crystallizer Based on: Properties of compound (solubility, temperature dependence) Crystallization process Required product specifications May also use: – – – – Fines removal Clear liquor Product removal Recycle loops Design of the Crystallizer From another process: o Batch process at 5 C for 12 hours Zinc chloride added to initiate crystallization – insulin6- Zn2 stoichiometry 0.5m3 reactor: 12 kg insulin to 11.31 kg of crystal (~95%) Proposed Design Seeded batch reactor with mixer Use 1 reactor OR multiple batches to create more continuous process – 17 mini-batches of 316 L per day from ultrafilter(Andrea) V V Q0 massrateinsulinout densityinsulin = residence time of crystals V = volume Qo = flow rate out Calculating V’s and Batch Times Their Process: 11.31kg/12 hr batch 0.5 m3 reactor volume Residence time gives 95% recovery Our Process: 188kg/batch with 98% recovery 1 batch for 6 h V = 17.04m3, C = US$239 500 1 b for 12 h V = 8.52 m3, C = US$156 200 2 b for 12 h V = 4.26 m3 each, C = US$105 300 3 b for 12 h V = 2.84 m3 each, C = US$85 200 Suppliers Alaqua, Inc. Ellett Industries, Ltd. GEA Evaporation Technologies Hosokawa Bepex Corp. Ionics Novatec, Inc. Walton/Stout, Inc. Resources Conservation Co., Div. Of Ionics Inc. Sulzer Chemtech USA, Inc. Swenson Technology, Inc. USFilter USFilter / HPD Products LIST, Inc. Questions? References •Bioprocess Design: http://cheserver.ent.ohiou.edu/ChE482/MoreBiosepExamples.pdf http://cheserver.ent.ohiou.edu/ChE482/biosep-examples.pdf •http://www.cheresources.com/cryst.shtml •http://www.tsk-g.co.jp/en/tech/uni/unil