Oral Presentation 4
advertisement

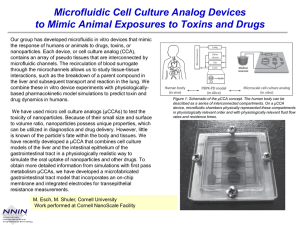
Group 3: Microfluidic System for Galvanotaxis Measurements Team Members: Arunan Skandarajah and Devin Henson Advisors: Dr. Janetopoulos, Dr. John Wikswo, and Dr. Paul King Physiological Presence of Electrical Fields • Normal development and maintenance processes result in endogenous electric fields1,2 – Established in wounds- ~.4-2 V/cm – Generated across gland and organ walls – Present during fetal organization • Result from ion flow across barriers Cells Respond to Electric Fields • Endothelial cells in wounds can be manipulated to speed or slow healing1 • Neuronal cells grow along electrically generated paths1 • Metastasizing cells will respond in the opposite manner of less malignant cells3 • Our model organism, Dictyostelium discoideum, also undergoes electrotaxis at voltages from 2-20V/cm4 Problem Statement How can we make experimentation easier than is currently possible? Forrester, 2007 (2) Issues with current technology 1. Difficult to fit bulky system on microscope stage 2. Exposed and electrified liquid dangerous to experimenters 3. Voltages as high as 500 V must be applied across device. 4. Excessive media and reagents required Solution Process • Utilize Microfabrication to – Reduce size of overall device to the scale of centimeters • Allow for easy use on microscope stage • Reduce the required applied voltage to ~50 V by reducing field size • Reduce the volume of cells and media required – Create a closed design • Perfuse Media through Device – Control of ion and pH gradients – Avoid problems with agar • Eliminate voltage drop resulting from long bridges Microfluidic Design Experimental Cell Area Resistance Distribution Calculations • DB Buffer = 552.49 Ω*cm • Conductivity electrode meter from the Cliffel Lab in SC5516 • 2% agar = 650 Ω*cm with considerable variation • Voltage drop due to electrolysis of water prevents precise measurement Current Status • • • • Microfabrication and AutoCAD training complete Prototypes produced Cell successfully cultured and seeded into devices Cell motility captured, but stated rates of movement have not been matched • Must establish baseline cell response to evaluate device before proceeding further Cells seeded in device, imaged at 32x using Zeiss Axiovert microscope and QImaging camera Current Status • Two macro-scale devices for establishing cell response 1. Coverslip method- replica from literature - easy to seed and clean - does not resolve any targeted problems 2. Microfluidic- “halfway” - lower voltage, better setup - more difficult to clean and seed Agar bridge access holes Agar bridge contact area glass slide PDMS Mcrofluidic channel Coverslip roof Preliminary Cell Motility Data • Cells are moving at approximately 1 μm/min • This value is 1/3 of that reported in literature4 • Poor gas exchange? Recent Developments • Reached Zhao to discuss protocol for macro-scale device – Reduce chamber length for better gas exchange (1 cm and 5 mm lengths) – Possible visit to UC-Davis • Received films for fabrication of microfluidic designs • Need to confirm new microfluidic devices work similarly to previous iterations for controlling pH and ion gradients – Find optimal flow rates and cell seeding methods Immediate Tasks • Try Zhao’s suggestions with shorter chamber lengths • Characterize flow profile and pH gradients in new microfluidics • Edit Kevin Seale’s code for our cell type and phase contrast imaging • Understand baseline response of cells in electric field • Select among prototypes for optimal microfluidic channel dimensions ImageJ Cell Tracking Software • Requires 8-bit images – QCapture capable of capturing in 8-bit – Previously captured 12-bit images are convertible to 8-bit no lost images • Obtained code for finding and tracking cells (Kevin Seale, SyBBURE) • Other macros for specific cell tracking downloadable online Future Direction • Utilize optimal microfluidic design to replicate cell response from literature • Improvements in using our device – Lower applied voltage necessary – Controlled pH and ion gradients without agar – Reduced overall device size and reagent consumption • Quantify data – Program ImageJ to obtain cell displacement – Find directedness and trajectory rates Works Cited 1. Nuccitelli, R., A role for endogenous electric fields in wound healing. Current Topics in Developmental Biology, 2003. 58: p. 1-26. 2. Forrester, J.V., Lois, N., Zhao, M., McCaig, C. The spark of life: the role of electric fields in regulating cell behaviour using the eye as a model system, Ophthal. Res. 39:4-16, 2007. 3. Pu, J., McCaig, C.D., Cao, L., Zhao, Z., Segall, J.E., Zhao, M. EGF receptor signaling is essential for electric-field-directed migration of breast cancer cells, J. Cell Sci. 120:3395-3403, 2007 4. Zhao, M., Jin, T., McCaig, C.D., Forrester, J.V., Devreotes, P.N. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis, J. Cell Biol. 157:921-927, 2002. .