Thermal Equilibration between 2 things : “Review”
advertisement

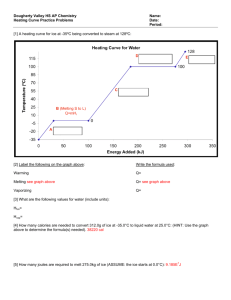
Thermal Equilibration between 2 things : “Review” Always assume 1 atm pressure, unless stated otherwise. 1. 2. No possible phase changes. QH + QC = 0. Solve for Tf Possible phase changes. Divide the process up into steps, each step involving adding heat to the cooler stuff and removing it from the warming stuff. There will always be two things that could end each step -- the one requiring less heat transfer will be the actual terminator. Terminate the step by transferring the smaller heat, recompute the composition and T of the cool stuff and the warm stuff, and repeat, until no further phase changes can occur. Example 1. Add 1 kg oil at 20°C to 1 kg water at 50°C. Final temp? • Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? What is the FIRST STEP in the approach to thermal equilibrium? A] ice melts as steam condenses B] ice melts as steam cools C] ice warms as steam condenses D] ice warms as steam cools Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? • Answer C: ice warms as steam condenses 1 kg•ci•(10°C) = 21 kJ 1 kg•Lv = 2256 kJ What terminates this first process? A] ice reaches 0°C B] ice reaches 100°C C] steam reaches 0°C D] all steam condenses E] it never terminates Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? • • Answer C: ice warms as steam condenses Answer A: ice reaches 0°C 1 kg•ci•(10°C) = 21 kJ 1 kg•Lv = 2256 kJ How much steam has condensed when the ice reaches 0°C (in kg)? A] none 2256 21 B] 2256 C] 21 2256 D] all of it Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? • • • Answer C: ice warms as steam condenses Answer A: ice reaches 0°C Answer C: Q for first step was 21 kJ. 2256 kJ would condense 1 kg (all) of the steam, so 21 kJ condenses 21/2256 kg of steam. What is the second step in thermal equilibration? A] ice melts while new water cools B] ice melts while more steam condenses C] ice warms while more steam condenses D] ice warms while new water cools Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? • • • • Answer C: ice warms as steam condenses Answer A: ice reaches 0°C Answer C: Q for first step was 21 kJ. 2256 kJ would condense 1 kg (all) of the steam, so 21 kJ condenses 21/2256 kg of steam. Answer B: ice melts while more steam condenses 1 kg•Lf = 334 kJ. What terminates the second step? A] all the steam condenses B] all the ice melts C] ice warms to 100°C D] steam cools to 0°C E] water cools to 0°C Example 2. Add 1 kg ice at -10°C to 1 kg steam at 100°C. Final temp? • Answer C: ice warms as steam condenses • Answer A: ice reaches 0°C • Answer C: Q for first step was 21 kJ. 2256 kJ would condense 1 kg (all) of the steam, so 21 kJ condenses 21/2256 kg of steam. • Answer B: ice melts while more steam condenses • Answer B: all the ice melts. 334 kJ < (2256-21) kJ. 1 kg•Lf = 334 kJ. Next you would calculate how much steam is left; then add heat to the water until all the steam is gone (IF it all condenses!) Radiation. Hot objects emit light (glowing red hot, white hot, light bulb filaments, etc.) dQ 4 AeT dt Room temp: infrared emission e = emissivity, e=1 is a ‘BLACK BODY’ Very hot: white emission VV hot: blue We can measure (approximately) the temperature of an object by looking at its black body spectrum. (This assumes that emissivity is independent of wavelength, which is often nearly true.) If an object can emit EM radiation (light, or infrared or UV etc.), then it must also absorb EM radiation. Consider an object in a vacuum box with perfectly reflective walls at temperature T. In thermal equilibrium, the object is emitting radiation: dQ 4 AeT dt Since it does not change temperature, it must absorb the same amount of radiation. So e= emissivity = absorptivity! Black objects heat up faster in the sun, but cool off faster at night!