MS Word
advertisement

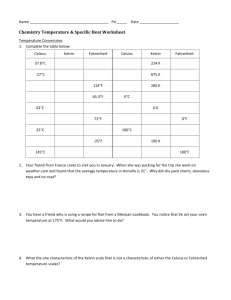
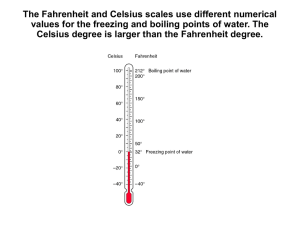
S369 Physics Phase Changes Use your textbook to answer the following: Name: 1. How many calories are needed to change 1 g of ice at 0 C to water? 2. How many calories are needed to change the temperature of 1 g of water by 1 C? 3. How many calories are needed to change 1 g of water at 100 C to steam? Now use this info to figure these out: 4. How many calories are needed to melt 1 g of ice and raise its temperature to 23 C? 5. A 50g sample of ice at 0 C is placed in an insulating container holding 200 g of water at 20 C. a. How much heat is necessary to melt the ice? b. If the water provides this heat, by how much would the temperature of the water change? c. What will the final temperature of the mixture be? 6. Fill in the calories required at each step: in changing from ice to steam: 1 gram ice at 0 C 1gram of water at 0 C 1 gram water at 100 C 1 gram steam at 100 C 0 C ____ cal + ____cal + 100 C ____cal = _____cal 7. One gram of steam at 100 C condenses and cools to 22 C a. How much heat is released during condensation? b. How much heat is released when the water cools from 100 C to 22 C? c. How much total heat is released? 8. How many calories are given off by a car radiator that condenses 1.5 kg of steam at 100 C to water at 90 C?