Naming Compounds
advertisement

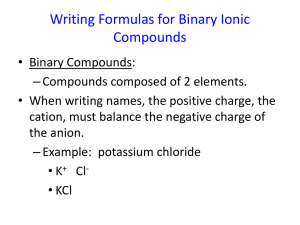
Naming Compounds *Binomial Nomenclature Two types of chemical compounds you will name: Binary Ionic: Metal and a nonmetal We will not be using transition metals! Binary AKA: Molecular: Two nonmetals Covalent compounds REMINDER: Metals: Are found to the LEFT of the stair-step line Remember, Nonmetals: Hydrogen is a nonmetal Are found to the RIGHT of the stair-step line Transition Metals: Found in the “dip” or middle of the periodic table 4 NAMING BINARY IONIC COMPOUNDS When naming, metals ALWAYS come before the nonmetal Name the first element as is Name the second element ending in “ide” EXAMPLES NaCl sodium chloride CaO calcium oxide Al2S3 MgI2 aluminum sulfide magnesium iodide NAMING BINARY MOLECULAR These compound contain two elements (binary) The term molecular indicates that these elements are joined by a covalent bond Two nonmetals Common Prefixes for Covalent Bonds Prefixes are used to tell the number of atoms of each element in a molecule Prefix Number mono di tri tetra penta hexa hepta octa nona deca 1 2 3 4 5 6 7 8 9 10 Naming Covalent Bonds You will use the prefixes to name covalent bonds Exception: DO NOT use mono before the first element if there is only one WRONG: CO- monocarbon monoxide CORRECT: Change CO- carbon monoxide the ending of the second element to “ide” EXAMPLES CO2 CO N2O4 CCl4 Carbon dioxide Carbon monoxide Dinitrogen tetraoxide Carbon tetrachloride WRITING FORMULAS FOR IONIC COMPOUNDS List the symbol for each ion and its charge 1. Ie Al3+ F- Find the least common multiple of the ion’s charges to make the compound Neutral 2. Least common multiple of 3 and 1 is 3 You need 1 Al and 3 F Write the formula with subscripts 3. AlF3 IONIC FORMULAS CRISS-CROSS METHOD Write the ions 2. Cross the charges down 3. Drop the charges 4. Reduce if necessary 1. EXAMPLES Lithium Oxide Potassium Barium Chloride Bromide Li2O KCl BaBr2 FORMULAS FOR MOLECULAR COMPOUNDS 1. 2. Write the symbols for the first and second element Translate the prefixes into subscripts EXAMPLES Diarsenic pentoxide As2O5 Tetraphosphorus trisulfide P4S3 Tetraphosphorus decoxide P4O10 Phosphorus trichloride PCl3