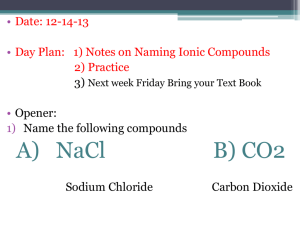
5.3 Compound Names and Formulas
advertisement
5.3-Compound Names and Formulas I can: -use the period table to determine period charge in Groups 1, 2, and 17, including hydrogen and oxygen -identify a compound using conventional naming systems that include Greek prefixes where appropriate (Prefixes limited to represent values from one to 10) -identify the formula for a covalent or ionic substance Naming Ionic Compounds ▪ Formed by cations-anions. ▪ Both ions are included in name. ▪ Naming of cations include the elements of which they are composed. ▪ Sodium loses an electron= Na+ ▪ Calcium loses two electrons= Ca2+ ▪ Aluminum loses three electrons= Al3+ – Group 1 ions have 1+ charge, while group 2 has 2+ charge Naming Ionic Compounds, cont. ▪ Names of anions are altered names of elements. – Same group will have same charge – “ide” is placed as suffix ▪ NaF=sodium fluoride Same cation names must show their charge ▪ FeO v. Fe2O3 – Both named iron oxide – See the problem? ▪ Transition metals can form several cations with different charges. ▪ Some cation name must be followed by Roman numeral in parentheses. – Fe2O3 = Fe3+, Iron(III) oxide. – now where did the Fe3+ come from? Determining the charge of a transition metal cation. ▪ Total charge must be 0! ▪ https://www.youtube.com/watch?v=_w6-4fRQt1Y Writing Formulas for Ionic Compounds ▪ What is the formula for Aluminum Fluoride? – AlF3 Naming Covalent Compounds ▪ SiO2 (silicon dioxide) and CO2 (Carbon Dioxide) ▪ Where are they on the periodic table? ▪ Numerical (#’s) prefixes are used to name covalent compounds of two elements – Tells how many atoms of each element – 1 atom in first element does not get prefix – Whichever element is further right on periodic table is named second and ends in “ide.” – In other words, start with one that is more metal like. ▪ BF3 – Boron trifluoride ▪ N204 – Dinitrogen Tetroxide ▪ CO? Chemical Formulas for Covalent Compounds ▪ Mass of each element in compounds must be measured first. ▪ Empirical Formula (simplest): Tells smallest whole-number ratio of atoms that are in compound. (reduced to ratio) – H2O= 2:1 ratio – Problem: compounds can have same empirical formula, but be very different. Molecular formulas are determined from empirical formulas. ▪ Each compound has its own Moleculelar formula: chemical formula that shows the number and kinds of atoms in a molecule, but not the arrangement of atoms. (actual number) ▪ Look at examples on previous slide. practice ▪ http://www.sciencegeek.net/Chemistry/taters/Unit3BinaryNomencla ture.htm