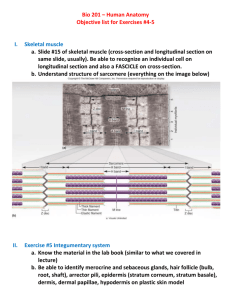
Transdermal drug delivery system
advertisement

Pharmaceutics 2 Unit 4 Transdermal drug delivery systems 1 • Transdermal drug delivery systems (TDDSs) facilitate the passage of therapeutic quantities of drug substances through the skin and into the general circulation for their systemic effects. • In 1965, Stoughton first conceived of the percutaneous absorption of drug substances. • The first transdermal system, Transderm Scop (Baxter), was approved by the Food and Drug Administration (FDA) in 1979 for prevention of nausea and vomiting associated with travel, particularly at sea. 2 • The stratum corneum, being keratinized tissue, behaves as a semipermeable membrane, and drug molecules penetrate by passive diffusion. • It is the major rate-limiting barrier to transdermal drug transport. • Once through the stratum corneum, drug molecules may pass through the deeper epidermal tissues and into the dermis. • When the drug reaches the vascularized dermal layer, it becomes available for absorption into the general circulation. 3 • The rate of drug movement across this layer depends on its concentration in the vehicle, its aqueous solubility, and the oil– water partition coefficient between the stratum corneum and the vehicle. • Substances with both aqueous and lipid solubility characteristics are good candidates for diffusion through the stratum corneum, epidermis, and dermis. 4 Routes of penetration • The diffusant has three potential entry routes to the viable tissue: • Through the hair follicles with their associated sebaceous glands, • Via the sweat ducts; • ( Transcellular or Intercellular) across the continuous stratum corneum between these appendages 5 Routes of drug absorption through skin 6 6 Skin appendages • Their fractional area available for absorption is small (about 0.1%) and this route usually does not contribute appreciably to the steady-state flux of a drug. • However, the route may be important for ions and large polar molecules that cross intact stratum corneum with difficulty. • For electrolytes and large molecules with low diffusion coefficients, such as polar steroids and antibiotics, and for some colloidal particles, the appendages may provide the main entry route. 7 the slope = - P Epidermal route • The epidermal barrier function resides mainly in the stratum corneum. • The corneocytes, consisting of hydrated keratin, are embedded in the in a complex lipid mixture of ceramides, fatty acids, cholesterol and cholesterol esters, formed into multiple bilayers. • Most molecules penetrating through the skin use this intercellular microroute. 9 10 • Simplified diagram of skin structure and routes of drug penetration, • (a) Macroroutes: (1) via the sweat ducts; (2) across the continuous stratum corneum; (3) through the hair follicles with their associated sebaceous glands, • (b) Representation of the stratum corneum membrane, illustrating two possible Microroutes for permeation ( Transcellular or Intercellular) . 11 FACTORS AFFECTING PERCUTANEOUS ABSORPTION • Not all drug substances are suitable for Transdermal delivery. • Among the factors playing a part in percutaneous absorption are the physical and chemical properties of the drug, including its molecular weight, solubility, partitioning coefficient and dissociation constant (pKa), the nature of the carrier vehicle, and the condition of the skin. 12 Physicochemical factors • • • • • • Skin hydration. Temperature and pH. Diffusion coefficient Drug concentration Partition coefficient Molecular size and shape 13 Drug concentration • Drug concentration is an important factor. • Generally, the amount of drug percutaneously absorbed per unit of surface area per time interval increases with an increase in the concentration of the drug in the TDDS. 14 the area and time of application • The larger the area of application (the larger the TDDS), the more drug is absorbed. • Generally, the longer the medicated application is permitted to remain in contact with the skin, the greater is the total drug absorption. 15 solubility of the drug in both lipid and water • The drug should have a greater physicochemical attraction to the skin than to the vehicle so that the drug will leave the vehicle in favor of the skin. • Some solubility of the drug in both lipid and water is thought to be essential for effective percutaneous absorption. • the aqueous solubility of a drug determines the concentration presented to the absorption site, and the partition coefficient influences the rate of transport across the absorption site. 16 • Generally, drugs penetrate their unionized form. the skin better in • Non-polar drugs tend to cross the cell barrier through the lipid-rich regions (intercellular route), whereas the polar drugs favor transport between cells (transcellular route). • For example, erythromycin base demonstrates better percutaneous absorption than erythromycin ethyl succinate. 17 Drugs molecular weight • Drugs with molecular weights of 100 to 800 and adequate lipid and aqueous solubility can permeate skin. • The ideal molecular weight of a drug for Transdermal drug delivery is believed to be 400 or less. 18 Hydration of the skin • Hydration of the skin percutaneous absorption. generally favors • The TDDS acts as an occlusive moisture barrier through which sweat cannot pass, increasing skin hydration. 19 Temperature and pH • The penetration rate of material through human skin can change tenfold for a large temperature variation, as the diffusion coefficient decreases as the temperature falls. • Clothing on most of the body would usually prevent wide fluctuations in temperature and penetration rates. • Occlusive vehicles increase skin temperature by a few degrees, but any consequent increased permeability is small compared to the effect of hydration. 20 PH • Only unionized molecules pass readily across lipid membranes. So when weak acids and bases dissociate to different degrees, depending on the pH and their pKa or pkb values. Thus, the proportion of unionized drug in the applied phase mainly determines the effective membrane gradient, and this fraction depends on pH. 21 Partition coefficient • Polar cosolvent mixtures, such as propylene glycol with water, may produce saturated drug solutions and so maximize the concentration gradient across the stratum corneum. • However, the partition coefficient of a drug between the membrane and the solvent mixture generally falls as the solubility in the solvent system rises. • Thus, these two factors - increase in solubility and decrease in the magnitude of the partition coefficient - may oppose each other in promoting flux through the membrane, when the system is not saturated. • Hence it is important not to over solubilize a drug if the aim is to promote penetration: the formulation should be at or near saturation. 22 Biological factors • • • • • Skin condition. Skin age. Blood flow. Regional skin sites. Skin metabolism. 23 Skin condition • The intact, healthy skin is a strong barrier but many agents can damage it. • acids and alkalis injure barrier cells and thereby promote penetration, as do cuts, abrasions and dermatitis. • skins may lose their reactivity or 'harden' because of frequent contact with irritant chemicals. • Disease commonly alters skin condition; In diseases characterized by a defective stratum corneum ( e.g. psoriasis), percutaneous absorption usually increases. • permeability increases: the skin inflamed, with loss of stratum corneum and altered keratinization. • permeability decreases: the organ thickened, with corns, calluses and warts, 24 Skin age • It is often assumed that the skin of the young and the elderly is more permeable than adult tissue • Children are more susceptible to the toxic effects of drugs and chemicals, partly because of their greater surface area per unit body weight; thus potent topical steroids, boric acid and hexachlorophane have produced severe side-effects and death. 25 Blood flow • an increased blood flow could reduce the amount of time a penetrant remains in the dermis, and also raise the concentration gradient across the skin ( Sink condition ) . Regional skin sites • Variations in cutaneous permeability around the body depend on the thickness and nature of the stratum corneum and the density of skin appendages. 26 Skin metabolism • The skin metabolizes steroid hormones, and some other drugs. • Such metabolism may determine the therapeutic efficacy of topically applied compounds (particularly prodrugs) 27 PERCUTANEOUS ABSORPTION ENHANCERS • Increase percutaneous absorption of therapeutic agents: 1.chemical permeation enhancers 2.physical methods 28 CHEMICAL ENHANCERS • increases skin permeability by reversibly damaging or altering the physicochemical nature of the stratum corneum to reduce its diffusion resistance • Among the alterations are: • increased hydration of the stratum corneum, • a change in the structure of the lipids and lipoproteins in the intercellular channels through solvent action or denaturation, or both 29 skin penetration enhancers • Water • Sulphoxides (especially dimethylsulphoxide) and their analogues • Pyrrolidones • Fatty acids and alcohols • Azone and its derivatives • Surfactants - anionic, cationic and non-ionic • Urea and its derivatives • Alcohols and glycols • Essential oils, terpenes and derivatives • Synergistic mixtures. 30 • The selection of a permeation enhancer should be based on: • its efficacy in enhancing skin permeation • its dermal toxicity (low) • its physicochemical and biologic compatibility with the system’s other components 31 Physical methods • Iontophoresis and Sonophoresis. • Iontophoresis is delivery of a charged chemical compound across the skin membrane using an electrical field. A number of drugs have been the subject of iontophoretic studies; they include Lidocaine; dexamethasone; amino acids, peptides, and insulin ; Verapamil; and propranolol. • Sonophoresis, or high-frequency ultrasound, Among the agents examined are hydrocortisone, lidocaine, and salicylic acid in such formulations as gels, creams, and lotions. It is thought that high frequency ultrasound can influence the integrity of the stratum corneum and thus affect its penetrability. 32 33