Transdermal drug delivery systems
advertisement

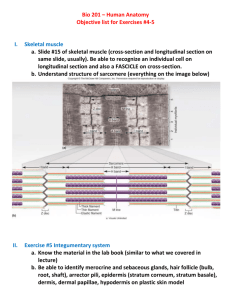
Topical drug delivery Karunya Kandimalla, Ph.D. Assistant professor, Biopharmaceutics and drug delivery E-mail: karunya.kandimalla@famu.edu Office phone: 850-599-3581 Skin: Functions With a thickness of only 3mm skin performs 1. Containment function 2. Protective function • Microbial barrier • Chemical barrier • Radiation barrier • Mechanical barrier • Heat barrier 3. Temperature regulation The skin: microscopic structure Hair follicle Sebaceous gland Stratum corneum (0.8mm – 0.006mm) Epidermis Dermis (3 – 5mm) Hypodermis Hair matrix Endocrine sweat gland Stratum corneum: transdermal drug delivery barrier Transport Pathways The drug permeation pathways across the SC include: 1. Intercellular pathway: across the lipid matrix 2. Transcellular pathway: across keratinized cells 3. Transappendageal pathway: across hair follicles and sweat ducts Intercellular and transcellular pathways are only accessible to non-polar molecules Transappendageal pathway is accessible to polar molecules it occupies only 0.1% of the skin surface area. Hence its contribution as a drug transport route is limited Approaches to topical treatment (1) (2) (3) (1) Manipulate the barrier (2) Direct drugs to viable skin tissues (3) Skin treatment for systemic conditions Target regions of topical treatment Interfacial boundries (1) Surface (2) Stratum corneum Penetration routes Drug dissolves diffuses, releases from vehicle Partition/diffusion stratum corneum (3) Appendages Pilosebaseous unit Some treatments 1. Camouflage 2. Protective layer 3. Insect repellant 4. Antimicrobial 1. Emoliency 2. Keratosis Ecrine 1. Antiperspirant gland 2. Exfolient 3. Antibiotic 4. Depilatory Interfacial boundries Penetration routes Some treatments (con’t) (4) Viable epidermis Partition/diffusion viable epidermis 1. Antiinflammatory 2. Anaesthetic Dermis corneum (5) Circulation Partition/diffusion dermis Removal via circulation 3. Antipruritic 4. Antihistamine 1. Transdermal delivery Transdermal drug delivery systems (TDDS) • Pharmaceutical formulations that are designed to deliver an active drug across the skin into systemic circulation. • Substances that possess both aqueous and lipid solubility characteristics are good candidates for diffusion through skin. • Types of transdermal control released systems. 1. Membrane controlled systems. 2. Adhesive diffusion - controlled systems. 3. Matrix controlled systems. TDD Patch Construction Matrix Nitro-Dur (Key Pharma) Reservoir E.g.Transderm-NitroTM (Ciba/Pharmaco) Drug-in-Adhesive Multi-Layer DeponitTM (Pharma-Schwartz) Backing Drug Drug-in-Adhesive Single-Layer Nitrodisc (Searle Pharma) Membrane Adhesive Liner/Skin Currently marketed TDDS Drug Applications Scopolamine Nitroglycerin Estradiol Clonidine Testosterone Isosorbide dinitrate Fentanyl Nicotine Estradiol/ Norethisterone Ac. Estradiol/Norethindrone Motion Sickness Angina Pectoris Post menopausal symptoms Hypertension Hypogonadism Angina pectoris Pain Smoking cessation Hormone deficiency Hormone deficiency and Post menopausal symptoms Birth control Norelgestromin and ethinyl estradiol Topical preparations 1. Retinoic acid (acne) 2. Tetracycline (sores) 3. Corticosteroids (psoriasis) 4. Bacitracin (eczyma) 5. Coal tar extracts (contact dermatitis) 6. Cromatin (scabies) 7. Zinc oxide (general healing) Why transdermal drug delivery? • Continous IV administration at a constant rate of infusion is a superior mode of drug delivery • IV administration avoids hepatic first-pass metabolism and maintain constant therapeutic drug levels in the body • TDD can closely duplicate continuous IV fusion. Hence it is helpful in delivering drugs that undergo significant first pass metabolism and/or have narrow therapeutic index Principles of diffusion through membranes Homogenous membrane Aqueous pores Cellulose fibres (1) Diffusion - random molecular motion. Must have concentration gradient. K D C0 Donor solution h Cd C1 C2 Donor Cr Receptor Fick’s law of diffusion dM SDC1 C2 J dt h Since K = C1 = C2 Cd Cr Where, dM = change in mass transferred dt in change of time t D = diffusion constant C1 = concentration in donor compartment C2 = concentration in receptor compartment S = surface area of membrane dM SDK Cd Cr dt h Under sink conditions and rearranging all constants, M r PSC d t Where, P = permeability constant Complex diffusional barriers Stratum corneum Epidermis Dermis Subcutaneous 1 1 1 1 R ... t P DK D K D K t 1 1 2 2 n n Where, Rt = Total diffusing resistance Pt = Thickness – weighted permeability coeff. Parrallel dM SDK Cd Cr dt h FOR EACH Factors influencing the rate of percutaneous diffusion 1. Diffusant solubility (C0) 2. Partition coefficient (K) 3. pH variation (K) 4. Co-solvents (K and C0) 5. Surface activity and micellization (C0) 6. Complexation (K) 7. Diffusivity (D) Factors that effect percutaneous absorption Biological factors 1. Skin age 2. Skin condition 3. Regional skin sites 4. Skin metabolism 5. Circulatory effects Physicochemical factors 1. Skin hydration 2. Drug/skin binding 3. Temperature 4. Penetration enhancers 5. Drug/vehicle interaction Attributes of a Passive TDD Drug Candidate • • • • • • Daily dose (< 20 mg/day) Half-life (10 hours or less) Molecular weight (< 500 Daltons) Melting point (< 200 oC) Skin permeability Lipid solubility [partition coefficient (Log P) between –1.0 and 4] • Toxicology profile (non-irritating and non-sensitizing to skin) Factors Important for Transdermal Drug Delivery • Physical and chemical properties of drug • Molecular weight, solubility, partitioning coefficient and pKa • Nature of the carrier vehicle • Condition of the skin. • Drug concentration is a important factor. The amount of drug percutaneously absorbed per unit of surface area per time interval increases as the concentration of the drug substance in the TDDS is increased • More drug is absorbed through percutaneous absorption when the drug is applied to a larger surface area (e.g. a larger size TDDS). Requirements..contd. • The drug substance should have a greater physicochemical attraction to the skin than to the vehicle in which it is presented. Solubility of drug in both lipid and water is though to be essential for percutaneous absorption. • The aqueous solubility of a drug determines the concentration presented to the absorption site and the partition co-efficient influences the rate of transport across the absorption site. Drugs penentrate the skin better in its unionized form. Non-polar drugs tend to cross the cell barrier through the lipid rich regions (transcellular route) whereas polar drugs favor transport between cells (intercellular route). Requirements…. • Drugs with molecular weight between 100-800 with adequate lipid and aqueous solubility can permeate skin. The ideal molecular weight of a drug for transdermal delivery is 400. • The skin hydration favors percutaneous absorption. TDDS act as occlusive moisture barriers through which the sweat from the skin cannot pass resulting in increased skin hydration. • In General, the longer the time the medicated application is permitted to remain in contact with the skin, the greater will be the drug absorption. • In cases the skin is abraded or cut will permit drugs to gain direct access to subcutaneous tissues and the capillary network obviating the designed function of the TDDS. Skin permeation enhancement • Physical approach – – – – Stripping of stratum corneum Hydration of stratum corneum Iontophoresis or phonophoresis Thermal energy • Chemical approach – Synthesis of lipophilic analogues – Delipidization of stratum corneum – Coadministration of skin permeation enhancer • Biochemical approach – Synthesis of bioconvertible prodrugs – Coadministration of skin metabolism inhibitors. Skin penetration enhancers Agents that enhance skin permeability. They act by: – Disruption of the highly ordered stratum corneum lipids – Interaction with cellular proteins – Improved partitioning of drug co-enhancer or cosolvent into the stratum corneum. Skin penetration enhancers Contd … The amount of drug transported through unit area of skin per unit time (Flux, J) is the product of diffusion coefficient of drug in the skin (D), the skin-vehicle partition coefficient (K) and the drug concentration in the vehicle or delivery system (C), divided by the thickness of skin (h). Flux (J) = (DKC)/h Skin penetration enhancers Contd … In principle enhancers act by: • Increasing drug partitioning (DK) in the stratum corneum by acting as solvents to dissolve the skin lipids or to denature skin proteins. • Increasing the drug solubility (C) in the transdermal formulation / patch S. Chemical class of Examples No enhancer 1 Fatty acids Oleic acid, Lauric acid 2 Fatty acid esters Isopropyl myristate, Isopropyl palmitate, 3 Fatty alcohols Olyl alcohol, Lauryl alcohol 4 Fatty alcohol ethers -Monoglyceryl ether 5 Azone and related Azone (laurocapram), NDodecyl-2-pyrrolidone, compounds 6 Complexing agents Cyclodextrins and their derivatives, HPMC 7 Pyrrolidones and 2-Pyrrolidone, N-Me-2pyrrolidone, Brij 36T, Tween 80, Cetrimide, Sodium lauryl sulphate related compounds 8 Classical surfactants 9. Dimethyl sulphoxide and related compds. Decylmethyl sulphoxide, Dimethyl sulphoxide 10. Ionic compounds Macrocyclics Sodium hyaluronate, Ascorbate, 11. 12. 13. Amines and Amides Solvents and related compds. Macrocyclic lactones, Ketones, Anhydrides Olyl amine, Lauryl amine, Urea, Ethanol, Polyethylene glycol, Propylene glycol 14. Biologicals Lecithin, Sodium deoxychloate 15. Enzymes Papain, Acid phosphatase 16. Others Terpenes (Menthol, Eucalyptol, etc.), Cardamom oil, Anise oil, Euginol, Bisabolol,Cysteine HCl Penetration enhancers Enhance transport of polar drugs via; (1) Extraction of stratum corneum lipids, lipoproteins, and nucleoproteins. (2) Loosening of the polymeric structure of the keratinocyte of the cytoplasmic matrix. (3) Changing the solvent properties of the stratum corneum. Enhancement of skin transport Surfactants Enhance transport of polar drugs via; (1) Solubilization and removal of intercellular lipids. (2) Interaction and binding to keratin filaments of intracellular matrix resulting in disruption of cell order. Non passive transport? Compounds like disopropanolamine and isopropyl myristate may help transport drugs via active Mechanisms, i.e. carrier – mediated transport. cell membrane Cell interior Drug + Carrier Drug Carrier Carrier Carrier Drug O (CH2)11 - CH3 N 1- dodecylazocycloheptane – 2- one O CH3 N C CH3 N,N – dimethylformamide (DMF) H O CH3 N C CH3 CH3 N,N – dimethylacetamide (DMA) CH3 S O CH3 Dimethylsulphoxide (DMSO) N O 2- pyrollidone Methods for studying percutaneous absorption 11.1 In vitro methods 11.1.1 Without a rate limiting membrane stirrer stirrer Ointment Alcohol in water Ointment Chloroform sink support With a rate limiting membrane Donor compartment With formulation Membrane (cellulose acetate, Silicone, Isopropyl myristate) Sampling port Receptor compartment (mainly chloroform) Bar magnetic stirrer In vivo methods 11.2.1 Direct methods Measurement of Pharmacological response • Antihypertensives by means of measuring blood pressure • Antihistamines by means of measuring the reduction in swelling 11.2.2 Indirect methods • Transepidermal water loss • Thermal determinations • Analysis of body fluids