William Rosenbloom—Unit 7—2011—pd 3 Types of Radiation
advertisement

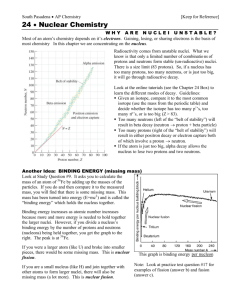
William Rosenbloom—Unit 7—2011—pd 3 Types of Radiation Alpha Radiation: emission of alpha particles, essentially helium nuclei, example: 238 234 4 92U → 90Th + 2He Beta Radiation: emission of beta particles, either electrons or positrons, operates through the basic mechanism of: 0 1 1 0n → 1p + −1e for electron emission and 0 1 1 1p → 0n + 1β for positron emission Gamma Radiation: emission of gamma rays, typically occurs as a byproduct of alpha decay or beta decay (note that only alpha decay changes an atom's mass number, and thus the change in an atom's mass number and its atomic number can be used to determine how many of each type of decay took place in it) Rate of Decay Radioactive isotopes are assigned a constant, k, that largely determines the rate at which the isotopes decay. The activity, A, in decays per unit of time, of a collection of N particles equals kN. The number of particles remaining, n, after some unit of time, t, can be found using the equation 𝑛 = 𝑁𝑒 −𝑘𝑡 . Using that equation the ln(2) half life of the given isotope can be calculated as 𝑘 . The equation for finding the activity, a, after some amount of time is found using the equation 𝑎 = 𝐴𝑒 −𝑘𝑡 . Stability, Binding Energy, and Mass Defect Looking at the trends of stable isotopes, they tend to be of very similar ratios of neutrons to protons. On a graph where x is the number of protons and y is the number of neutrons, nearly every stable isotope exists within a specific region called the "belt of stability." The belt of stability rises above the line y=x because, as the number of protons in a nucleus increases, more neutrons are needed to reduce the repulsion between them. No elements with an atomic number greater than 83 are stable. If an isotope is above the belt, and has too many neutrons, it will probably experience electron emission to increase its ratio of protons to neutrons. If an isotope is below the belt of stability it will experience positron emission to increase its neutron to proton ratio. If an isotope has more than 83 protons it will attempt to reduce its number of both protons and neutrons through alpha decay. The quantitative stability of an isotope is determined by finding its binding energy. Binding energy is related to an atom’s mass defect. The mass defect is a change in mass following a nuclear reaction. The mass is lost because it is converted into energy. The amount of energy released by a reaction’s mass defect is equal to the amount of mass lost multiplied by the square of the speed of light, as shown in the equation 𝐸 = 𝑚𝑐 2 . A proton and neutron both have a standard mass, but the mass of a nucleus is usually less than the mass of a proton multiplied by how many protons it has plus the mass of a neutron multiplied by the number of neutrons it has. The difference between what the atomic mass would be if none of the neutrons or protons lost mass and its real mass is considered a mass defect. The energy released by the mass defect of the nucleus is the nucleus’s binding energy. Much more relevant measure of binding energy per nucleon is found by dividing the binding energy by the number of neutrons and protons in the nucleus. This measure will determine the stability of the isotope.