The Photoelectric Effect
advertisement

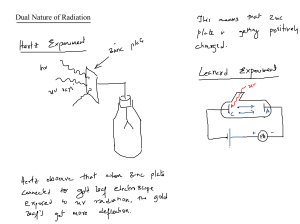
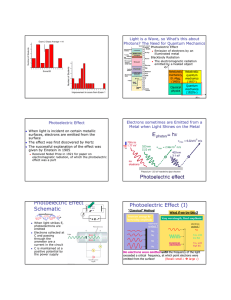
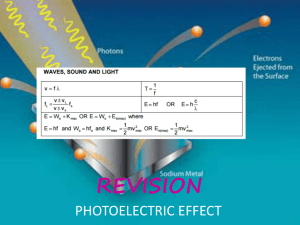
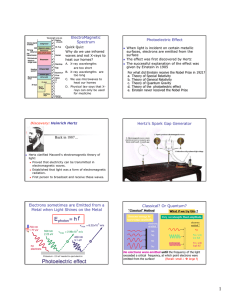
The Photoelectric E ffect By Eleanor Girdziusz The Photoelectric Effect “The phenomenon that when light shines on a metal surface, electrons are emitted” Wave Theory Supported by such observations as: • Refraction of light • Reflection of light • Interference patterns • Diffraction of light Wave Theory • Energy from radiation spread equally • Low intensity means no emission of electrons • Energies of the electrons do not increase with intensity of the radiation Quanta In 1901, Max Planck suggested light was made up of ‘packets’ of energy: E = hf h = Planck’s constant = 6.626x10-34Js f = frequency of radiation Photons • In 1905, Albert Einstein presented the idea that energy is not just emitted as quanta, but continues to exist in this form • Thus light is a stream of photons, each with energy hf Photoemission • The number of electrons emitted by a surface is proportional to the number of incident photons • An electron is emitted as soon as a photon reaches the surface hf = φ + ½ mV2 φ = work function ½ mV2 = max kinetic energy Threshold Frequency hf < φ hfo = φ hc = φ λo c = speed of light λo = wavelength Work Function The minimum amount of energy that has to be given to an electron to release it from the surface of the material and varies depending on the material Gold Leaf Electroscope Demonstration The Photoelectric Effect • UV light causes electrons to be released from the surface of the zinc • Emission of electrons commences at the instant the surface is irradiated • The frequency of the radiation must exceed the threshold frequency • Intensity of radiation has no effect