The Photoelectric Effect
advertisement

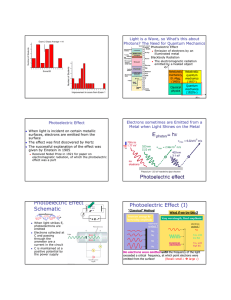
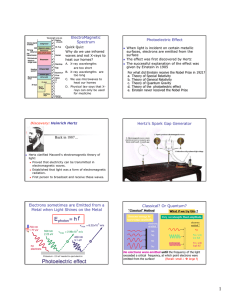
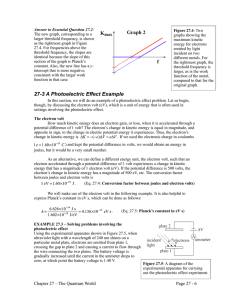
THE PHOTOELECTRIC EFFECT powerful evidence that light is particulate (behaves like little bullets) not explained by a wave theory When light in the form of photons (packets of discrete electromagnetic wave energy) falls on a metal surface, electrons are ejected from the surface. All the photon energy is given up to one electron Photon energy = hf Emission is instantaneous Excess energy over and above that required for ejection is picked up as kinetic energy of the moving electron Einstein’s Photoelectric Equation incident photon energy = energy required to overcome electrostatic attraction of surrounding nuclei (WORK FUNCTION , ) + maximum kinetic energy of the emitted electron. usually measured in eV (1eV = 1.6 x 10-1J) hf = + 1/2mv2max If f < a threshold frequency, fo no emission occurs no matter how bright the illumination (i e, more photons) The kinetic energy (speed) of the emitted electrons is variable. This is because electrons deeper in the lattice require more energy to get them out ( higher than those immediately at the surface. Emission depends on photon energy, (or frequency E=hf), NOT on intensity A graph of Ek against incident frequency is a straight line, intersecting the x axis at the THRESHOLD frequency, fo below which no emission can occur. The slope of this graph is h, the y-intercept is Page 1 of 3 THE PHOTOELECTRIC EFFECT Ek f When a positive potential is applied to the collector, which collects the emitted photoelectrons, a fraction of the total number emitted is collected. If the potential rises sufficiently, all the emitted electrons are collected. I Bright light Graphically: Dimmer light V Vs If a negative potential, Vs called the STOPPING POTENTIAL is applied, the photoelectric current will decrease to zero, for a given photon energy Decide with a reason, which metal has the higher work function by looking at the graph below. The two metals have been illuminated identically. I V Page 2 of 3 THE PHOTOELECTRIC EFFECT Problems. Check this applet out. http://physics.berea.edu/%7Eking/Teaching/ModPhys/QM/Photoelectric/Photoelectri c.html A particular metal has = 2.4 eV. What is the maximum wavelength of illumination required to eject an electron from its surface (recall c = fWhere is this light in the EM spectrum? Light of frequency 3 x 1015Hz falls on the surface. Find the max speed of the emitted electrons, also their energy in eV. (mass of an electron = 9x10-31 kg) Page 3 of 3