An overview of INSPIR - Boston University Medical Campus
advertisement

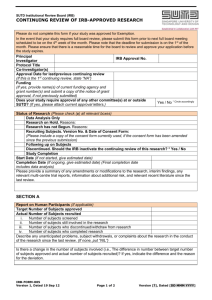
Introduction to INSPIR OCR Seminar Series January 21, 2004 Mary Banks, RN Administrator, Office of the IRB “The Future Is Now” Yogi Berra What is INSPIR ? Integrated Network for Subject Protection In Research INSPIR Background • Developed as BRAIN at Baylor • • • • • School of Medicine Currently used at Baylor and University of Maryland (BRAAN) Result of IRB Improvement Grant (NIH) Customized to fit Boston University requirements Will be used at BUMC and Charles River Campus Used by BUMC IRB and IACUC System Requirements • January Issue of CR Times • • (www.bu.edu/crtimes)- link Connecting to INSPIR Browser – Macintosh, Netscape version 7 – Safari for Mac – PC- Internet Explorer 6.0 or higher • Cookies must be enabled • Pop-up Blockers must be disabled www.bumc.bu.edu/Departments/PageMain.asp?Page=8725&DepartmentID=368 Useful Tip • Attachments (drug brochures, • sponsor’s protocol, grant applications) will have to be scanned and “attached” to the IRB application Extremely helpful if you have a scanner within the department to use to scan these documents Access to INSPIR • Website “Getting Access” • Presence in BU’s electronic • • • directory BU Login Name, BUID Number, and Kerberos password If you don’t have a presence in BU’s directory (BMC, VA) instructions available Do this now- don’t wait www.bumc.bu.edu/Departments/PageMain.asp?Page=8636&DepartmentID=368 Difficulties Logging Into INSPIR • Login issues and technical • • issues will NOT be managed by the IRB office Contact the IT INSPIR Team at BRAAN@bu.edu Helpful advice on website under “Help!” www.bumc.bu.edu/Departments/PageMain.asp?Page=8094&DepartmentID=368 What Does INSPIR Do? • Allows for IRB protocols to be created, • • • • routed, reviewed, signed, and approved within the system Provides for transparent processingeveryone who has access can see where the protocol is at each step in the process Creates IRB meeting agendas and minutes Gives reviewers and investigators access to the entire study file (including previous versions that are archived in the system) Automatically generates continuing review reminders Why Be INSPIRed? • Electronic submission of protocols – No longer need to submit multiple copies to IRB – Not rely on interdepartmental mail – No need to hand deliver documents to the IRB office – Documents won’t get lost – Accurate study files- investigator files will match IRB files Why Be INSPIRed? (cont.) • Investigators can track the progress of • • • • own protocols Multiple copies of documents (approval letters, consent forms, etc.) with version control can be printed Applications are automatically routed for required signatures Continuing Review process is much easier because amendments, SAEs, etc. are cumulatively tracked Investigators can access INSPIR from any internet connected computer 2004 Jan Feb summer April Mar Mar. 8 IRB office Stops Accepting Paper Mar. 15 GO LIVE 3/8 - 3/15 IRB office CLOSED 2/23 - 3/15 Train the Investigators May Important Dates for Investigators and Research Staff at BUMC • February 23 - March 15 • • • Investigator training March 8 IRB Office stops accepting paper submissions March 8 - 15 IRB Office closed March 15 Go Live Date Investigator Training • Feb. 23 - Mar.15 • Training will include – – – – Online training Investigator Manual Large group classes Small group classes • Schedule of classes will be • posted Practice site will be available for investigators to use IRB Office Stops Accepting Paper • March 8, 2004 • All paper Progress Reports must • • • be submitted by this date Can submit Progress Reports for March, April and May Last date to accept new Research Applications in paper Last date to accept amendments in paper IRB OFFICE CLOSED • March 8-15 • No paper or electronic • • submissions accepted during this week Investigator training will be ongoing during the week IRB office staff will be available for training and to answer questions GO LIVE • March 15, 2004 • First day protocols can be submitted electronically – New protocols – Progress Reports (Continuing Reviews) – Amendments New IRB Applications • Full Board, Expedited and • • • Exempt Submit on paper by March 8 OR Must hold and submit on or after March 15 electronically Investigators can work on drafts prior to March 15 Continuing Review • Progress Reports must be • • • submitted by March 8 on paper OR Must be held until March 15 for submission electronically Investigators may submit on paper for March, April, May only Studies that expire after May 31, 2004 – Progress Reports must be submitted electronically Continuing Review • After March 8 all Progress • Reports (CRs) must be submitted electronically To submit a Progress Report the investigator must first enter the study into the system using a new application Continuing Review • If PI submits a new protocol • • on March 8 on paper - will have to re-submit the study electronically at time of next Continuing Review Investigators must ensure that studies will not lapse Progress Reports must still be submitted at least 5 weeks prior to expiration Amendments • After March 8 all • amendments must be submitted electronically Prior to submitting an amendment the investigator must enter the protocol into the system (by completing a Research Application for the study) Serious Adverse Events • March 8 deadline does • • not apply to SAEs SAEs may be submitted on paper until the study is up for Continuing Review (or an amendment is needed) More information on SAEs will be coming - the IRB is modifying the policy on submitting SAEs to the IRB Demonstration of INSPIR • Demonstration site (practice • • • site) will be available soon for use by investigators Final modifications being made to the system We will provide investigators paper copies of screen shots We will provide an electronic Investigator’s manual Go To Demo