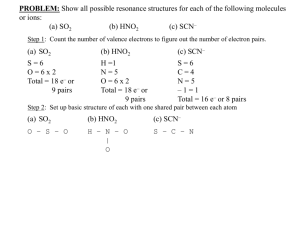
Finding the pH of a - BC Learning Network
advertisement

Finding the pH of a Weak Base Here we’ll go through the steps necessary to find the pH of a weak base with a given concentration. Find the pH of a 0.35 M solution of NaNO2. We’re asked to find the pH of a 0.35 M solution of the salt sodium nitrite, NaNO2. Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. A general approach to finding the pH of a salt solution is outlined here. We start by dissociating the salt into its individual ions and discarding any spectator ions. Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. 2. Identify remaining ions as acids or bases and as strong or weak. Next, we look at the remaining ion or ions and identify them as acids or bases and as strong or weak. We use location on the acid table for this. Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. 2. Identify remaining ions as acids or bases and as strong or weak. 3. If it is weak, write the hydrolysis equilibrium equation. If the ion acts as a WEAK acid or base we write its equilibrium equation for hydrolysis, the ion’s reaction with water. Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. 2. Identify remaining ions as acids or bases and as strong or weak. 3. If it is weak, write the hydrolysis equilibrium equation. 4. Using an ICE table, calculate [H3O+] or [OH–]. If it’s a weak acid, we use an ICE table and its Ka expression to calculate the hydronium ion concentration, Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. 2. Identify remaining ions as acids or bases and as strong or weak. 3. If it is weak, write the hydrolysis equilibrium equation. 4. Using an ICE table, calculate [H3O+] or [OH–]. and if it’s a weak base, we use an ICE table and its Kb expression to calculate the hydroxide ion concentration. Find the pH of a 0.35 M solution of NaNO2. 1. Dissociate the salt into it’s ions and discard any spectator ions. 2. Identify remaining ions as acids or bases and as strong or weak. 3. If it is weak, write the hydrolysis equilibrium equation. 4. Using an ICE table, calculate [H3O+] or [OH–]. 5. Find the pH. Finally we convert hydronium or hydroxide concentration to pH. Find the pH of a 0.35 M solution of NaNO2. Because NaNO2 is a salt, we’ll start by dissociating it into its individual ions. Find the pH of a 0.35 M solution of NaNO2. NaNO 2(aq) Na(aq) NO 2(aq) Neutral Spectator Which are Na+ and NO2 minus ions. Weak Base Find the pH of a 0.35 M solution of NaNO2. NaNO 2(aq) Na(aq NO ) 2(aq) Neutral Spectator Na+ is an alkali metal cation, so it is a neutral spectator. Weak Base Find the pH of a 0.35 M solution of NaNO2. NaNO 2(aq) Na(aq NO ) 2(aq) Neutral Spectator And we can discard it. Weak Base Find the pH of a 0.35 M solution of NaNO2. NaNO 2(aq) Na(aq) NO2(aq) ? In order to determine what NO2 minus acts as, we look for it on the acid table. Weak Base It’s at this location on the right side of the table, so it’s a weak base. NaNO 2(aq) Na(aq) NO2(aq) Weak Base Find the pH of a 0.35 M solution of NaNO2. Because NO2 minus is a weak base K b(NO ) 2 Kw K a(conjugate acid) Kw NaNO 2(aq) Na(aq) NO2(aq) K a(HNO2 ) 1.00 10 14 11 2.17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. We will need to find the value of its Kb Weak Base K b(NO ) 2 Kw K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2.17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. Remember the formula we can use for Kb of NO2 minus is Kb = Kw over the Ka of its conjugate acid. The conjugate acid of NO2– is HNO2 Looking at the table, we see that the conjugate acid of NO2 minus is HNO2. K b(NO ) 2 K b(NO ) 2 Kw K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2.17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. So the Kb of NO2 minus is Kw over the Ka of HNO2. Ka of HNO2 The Ka for HNO2 is shown on the table here, and its 4.6 × 10-4. K b(NO ) 2 K b(NO ) 2 Kw K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2.17 1 0 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. Now we can substitute. We know that Kw is 1.00 × 10-14, K b(NO ) 2 K b(NO ) 2 Kw K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2.17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. And the Ka of HNO2 is 4.6 × 10-4. K b(NO ) 2 K b(NO ) 2 Kw K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2. 17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. 1 × 10-14 divided by 4.6 × 10-4 comes out to 2.17 × 10-11. Even though the Ka we used has only 2 significant figures, we’ll express the Kb to 3 significant figures and round to 2 significant figures in the final answer. K b(NO ) 2 K b(NO ) 2 Kw Kb(NO ) 2.17 1011 2 K a(conjugate acid) Kw K a(HNO2 ) 1.00 10 14 11 2. 17 10 4 4.6 10 Find the pH of a 0.35 M solution of NaNO2. So we’ll make a note up here that the Kb of NO2 minus is 2.17 times 10-11. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO2(aq) OH(aq) Weak Base Find the pH of a 0.35 M solution of NaNO2. Because NO2 minus is a weak base, we can write its hydrolysis equilibrium equation. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO 2(aq) OH(aq) Find the pH of a 0.35 M solution of NaNO2. Hydrolysis means we add it to water. H+ NO 2(aq) H 2O( l ) K b(NO ) 2.17 10 11 2 HNO 2(aq) OH(aq) Weak Base Find the pH of a 0.35 M solution of NaNO2. Because it’s a base, it will accept a proton from water. K b(NO ) 2.17 10 11 H+ NO 2(aq) H 2O( l ) 2 HNO 2(aq) OH(aq) Find the pH of a 0.35 M solution of NaNO2. Forming HNO2 K b(NO ) 2.17 10 11 H+ NO 2(aq) H 2O( l ) 2 HNO 2(aq) OH(a q) Find the pH of a 0.35 M solution of NaNO2. And OH minus. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO 2(aq) OH(a q) ? Find the pH of a 0.35 M solution of NaNO2. On the way to finding pH, we can start by finding the hydroxide ion concentration. Because NO2 minus is a WEAK base, we do this using an ICE table. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO2(aq) OH(aq) [I] [C] [E] Find the pH of a 0.35 M solution of NaNO2. So we draw an ICE table underneath this equilibrium equation. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO2(aq) OH(aq) [I] [C] [E] Find the pH of a 0.35 M solution of NaNO2. Water is a liquid so we don’t include it in our calculations. We’ll colour the column below water blue. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Now we’ll fill in what we can in the initial concentration row. 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x 0 +x x Find the pH of a 0.35 M solution of NaNO2. The initial concentration of NO2 minus is the same as the initial concentration of NaNO2, and it’s equal to 0.35 molar, so we’ll write 0.35 in here. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x 0 +x x Find the pH of a 0.35 M solution of NaNO2. Before hydrolysis occurs, we can say that the concentrations of HNO2 and OH minus are both zero. K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO2(aq) OH(aq) [I] 0.35 [C] –x [E] 0.35–x 0 +x x Find the pH of a 0.35 M solution of NaNO2. Now, we’ll fill in the change in concentration row. 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x 0 +x x Find the pH of a 0.35 M solution of NaNO2. Because the concentrations of the products are zero, in order to compensate… K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. The reaction will move to the right. 0 +x x K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. So the concentrations of HNO2 and OH minus will both increase 0 +x x K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x 0 +x x Find the pH of a 0.35 M solution of NaNO2. We’re not given any values at equilibrium and these both have a coefficient of 1 in the equation, we’ll state that these both increase by x K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x 0 +x x Find the pH of a 0.35 M solution of NaNO2. Because the reaction is moving to the right, the concentration of the reactant, NO2 minus, will decrease, so we’ll write a minus sign here. K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Like the HNO2 and the OH minus, the coefficient on NO2 minus is 1, 0 +x x K b(NO ) 2.17 10 11 2 Moves to NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x the Right HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. So we can say that the concentration of NO2 minus goes down by x. 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Now we can fill in the equilibrium concentration row. 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. We’ll start with the OH minus. It will be 0 plus x 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Which is equal to x 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Similarly, the equilibrium concentration of HNO2 will be 0 + x 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Which is equal to x 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Now, we’ll look at the NO2 minus. It started out as 0.35 molar 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. And it went down by x, 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. So its equilibrium concentration is 035 minus x 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. So now we have the equilibrium concentrations of all species. 0 +x x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. Notice that the equilibrium concentration of OH minus is x. 0 +x x OH x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) [I] 0.35 [C] –x [E] 0.35–x HNO2(aq) OH(aq) 0 +x x Find the pH of a 0.35 M solution of NaNO2. We’ll make a note of that up here. 0 +x x OH x K b(NO ) 2.17 10 11 2 NO 2(aq) H 2O( l ) HNO2(aq) OH(aq) [I] 0.35 [C] –x [E] 0.35–x 0 +x x Find the pH of a 0.35 M solution of NaNO2. At this point, we need to solve for the value of x. 0 +x x HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. We start by using the hydrolysis equilibrium equation to write the Kb expression for NO2 minus, which is the concentration of HNO2 times the concentration of OH minus over the concentration of NO2 minus. HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb Weak x 0.35Base x2 Kb 0 .3 5 Find the pH of a 0.35 M solution of NaNO2. Remember, because we’re dealing with the hydrolysis of a weak BASE, the expression is called Kb rather than Ka. HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. Now we’ll insert equilibrium concentrations into the Kb expression in order to solve for x. HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. The concentration of HNO2 and OH minus are both equal to x, HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. so their product in the Kb expression is x times x, or x squared HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. And the equilibrium concentration of NO2 minus is 0.35 minus x, HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. so we’ll substitute that in here for the concentration of NO2 minus. HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Assume 0.35–x 0.35 Find the pH of a 0.35 M solution of NaNO2. In order to avoid a quadratic equation, we assume that 0.35 minus x is almost equal to 0.35. HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Assume 0.35–x 0.35 Find the pH of a 0.35 M solution of NaNO2. We know this assumption is valid because the Kb of NO2 minus is very small. That means the amount it deceases by will be very small compared to its initial concentration HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Assume 0.35–x 0.35 Find the pH of a 0.35 M solution of NaNO2. Using this assumption, we take the x out of the denominator HNO2 OH Kb NO 2 OH x K b(NO ) 2.17 10 11 2 x2 Kb 0.35 x x2 Kb 0.35 Find the pH of a 0.35 M solution of NaNO2. And we get that Kb is approximately equal to x squared divided by 0.35. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 OH x K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Rearranging this equation gives us x squared = 0.35 times Kb HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 OH x K b(NO ) 2.17 10 11 2 x 2 0.35 K b Take square root of both sides OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Taking the square root of both sides gives us x = the square root of 0.35 times kb HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 OH x K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Remember from our ice table, that x is equal to the hydroxide ion concentration at equilibrium. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 OH x K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. So we can say that the hydroxide ion concentration is equal to x, which is equal to the square root of 0.35 kb HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 OH x K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Or more simply, [OH-] is equal to the square root of 0.35 kb HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Now we’ll substitute 2.17 × 10-11 in for the value of Kb in the equation HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 K b(NO ) 2.17 10 11 2 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. 0.35 times 2.17 × 10-11 is equal to 7.595 × 10-12. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Taking the square root of 7.595 × 10-12 gives us 2.76 × 10-6. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Because we now have a value for the concentration of hydroxide, we add the unit M for molarity. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. pOH is the negative log of the hydroxide ion concentration, which is the negative log of 2.76 × 10-6. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. And that comes out to 5.559. We’ll stick with 3 significant figures here and round off to 2 in the last step. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. The pH of a solution is 14 minus the pOH, which in this case is 14 minus 5.559. HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.441 Find the pH of a 0.35 M solution of NaNO2. Which comes out to 8.441 . HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 8.44 Find the pH of a 0.35 M solution of NaNO2. Both the given concentration of 0.35 molar and the Ka for HNO2 we used from the acid table have 2 significant figures. So we round our final pH to 2 significant figures, or 2 decimal places, so its 8.44 HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 x 2 0.35 K b OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 pH 8.44 Find the pH of a 0.35 M solution of NaNO2. So now we have a final answer for the question. The pH of a 0.35 molar solution of NaNO2 is 8.44 . HNO2 OH Kb NO 2 x2 Kb 0.35 x x2 Kb 0.35 K b(NO ) 2.17 10 11 2 x 2 0.35 K b Low OH x 0.35 K b OH 0.35 2.17 10 11 OH 7.595 10 12 2.76 10 6 M pOH log 2.76 10 6 5.559 pH 14.000 5.559 pH 8.44 Find the pH of a 0.35 M solution of NaNO2. This is reasonable because NO2 minus is a weak base, so we would expect its pH to be above 7, but not really high because its Kb value is quite low at 2.17 × 10-11.