(Practice) Summer 2015 CHEM 2325 Exam 2
advertisement
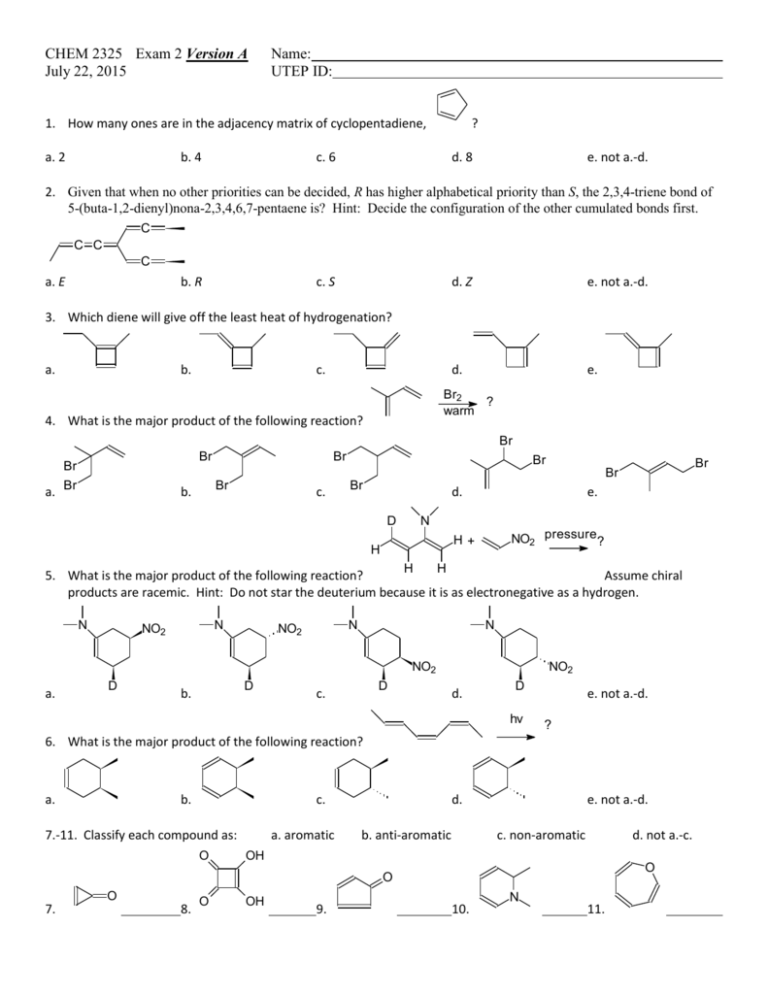
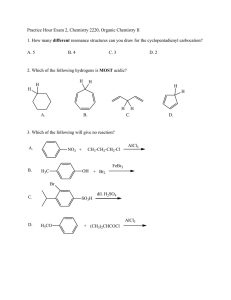
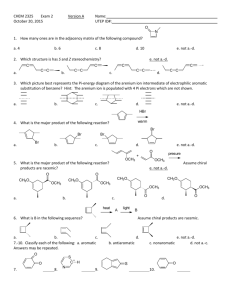
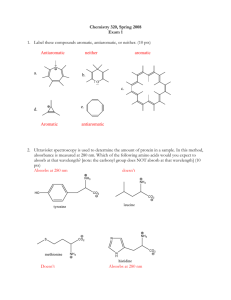
CHEM 2325 Exam 2 Version A July 22, 2015 Name: UTEP ID: 1. How many ones are in the adjacency matrix of cyclopentadiene, a. 2 b. 4 ? c. 6 d. 8 e. not a.-d. 2. Given that when no other priorities can be decided, R has higher alphabetical priority than S, the 2,3,4-triene bond of 5-(buta-1,2-dienyl)nona-2,3,4,6,7-pentaene is? Hint: Decide the configuration of the other cumulated bonds first. C C C C a. E b. R c. S d. Z e. not a.-d. d. e. 3. Which diene will give off the least heat of hydrogenation? a. b. c. Br2 ? warm 4. What is the major product of the following reaction? Br a. Br Br Br Br Br Br Br Br b. c. Br d. D e. N NO2 pressure? H+ H H H 5. What is the major product of the following reaction? Assume chiral products are racemic. Hint: Do not star the deuterium because it is as electronegative as a hydrogen. N N NO2 N NO2 N NO2 a. D D b. D c. NO2 d. D hv e. not a.-d. ? 6. What is the major product of the following reaction? a. b. c. 7.-11. Classify each compound as: O a. aromatic d. b. anti-aromatic e. not a.-d. c. non-aromatic d. not a.-c. OH O O O 7. 8. O OH 9. 10. N 11. a b d c 12. What is the longest bond of the following compound? e 13. Which compound reacts the slowest with Cl2/FeCl3? O OCH3 a. O b. OH O c. OH d. e. c b a d O e 14. Which position will be substituted in the major product of the reaction of H2SO4 and ? 15.-19. Match each reaction sequence to a major product on the right. Answers may be repeated. Assume any necessary workup between steps. Cl HNO3 Zn NaNO2 O H2SO4 15. HCl CuBr HCl, cold AlCl3 Br a. Cl N2H4 Br2 KOH, heat FeBr3 Br O 16. AlCl3 b. Cl HNO3 Br2 Zn NaNO2 HCl HCl, cold O H2SO4 17. c. AlCl3 Cl AlCl3 HNO3 H2 NaNO2 H2SO4 Pd HCl, cold HNO3 Br2 H2 NaNO2 Pd HCl, cold H2SO4 FeBr3 19. 20. Which reaction above uses a Wolff-Kishner reduction? a. question 15 Br AlCl3 Cl 18. FeBr3 H3PO2 b. question 16 CuBr Br d. c. question 17 H3PO2 Br e. d. question 18 e. question 19 The Exam 2 retake homework is due Friday, July 24, before 5:00 p.m. via http://organic.utep.edu/quiz. You may work together on this homework but everyone is responsible for submitting his or her own answers via the online form and you should not consult with anyone outside of class. Turn in your homework as long before the due date and time as possible. No excuses for late entries!