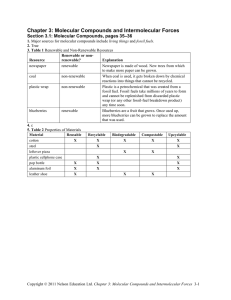
Name: Per: _____ Date: ______ Quiz – Polarity, Naming
advertisement
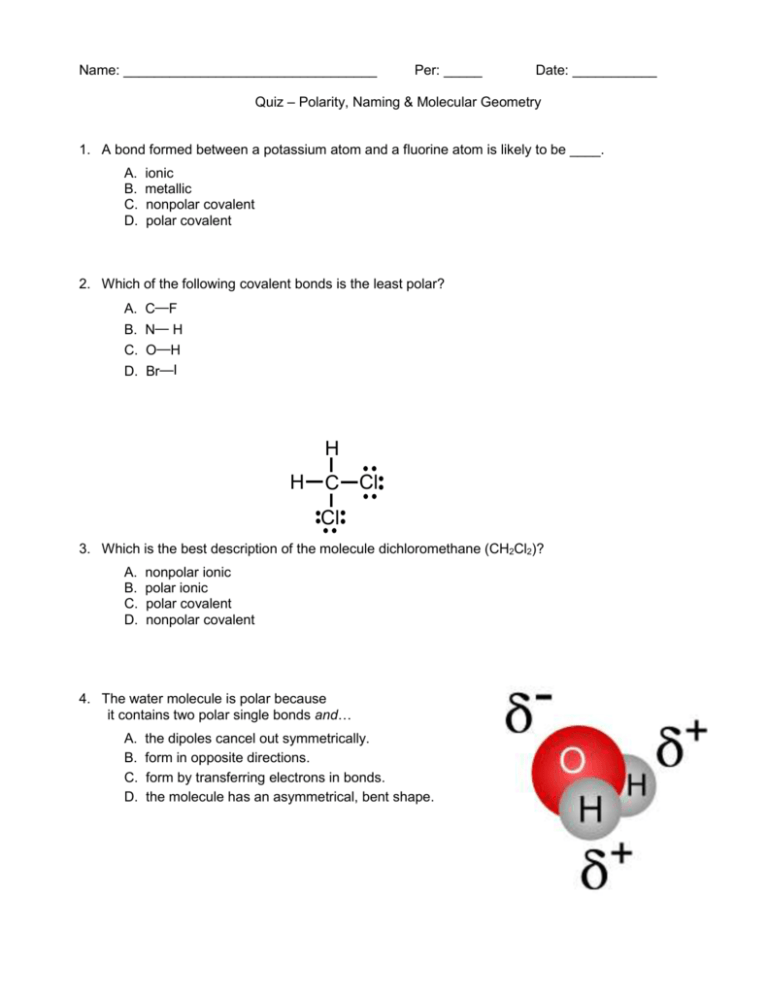
Name: _________________________________ Per: _____ Date: ___________ Quiz – Polarity, Naming & Molecular Geometry 1. A bond formed between a potassium atom and a fluorine atom is likely to be ____. A. B. C. D. ionic metallic nonpolar covalent polar covalent 2. Which of the following covalent bonds is the least polar? A. C—F B. N— H C. O—H D. Br—I H H C Cl Cl 3. Which is the best description of the molecule dichloromethane (CH2Cl2)? A. B. C. D. nonpolar ionic polar ionic polar covalent nonpolar covalent 4. The water molecule is polar because it contains two polar single bonds and… A. B. C. D. the dipoles cancel out symmetrically. form in opposite directions. form by transferring electrons in bonds. the molecule has an asymmetrical, bent shape. 5. What is the formula for phosphorus tetrafluoride? A. B. C. D. PF5 PF4 PF6 P7F 6. What is the formula for carbon monoxide? A. B. C. D. CO2 C2O2 CMO CO 7. Name the compound PH3. A. B. C. D. phosphorus trihydrogen phosphorus trihydride monophosphorus hydrogen phosphohydride 8. Name the compound Se2Cl6 . A. B. C. D. diselenium hexachloride diselenide hexachlorine selenium pentachlorine diselenium tetrachloride HONORS ONLY Questions 9-13 refer to the following four molecules. Draw the Lewis structure for each molecule. You may use the same answer more than once. (A) NH3 (B) CF2Cl2 (C) BH3 9. Which molecule has a trigonal planar molecular geometry? 10. Which molecule has bond angles of 180o ? 11. Which molecule has a tetrahedral molecular geometry? (D) HCN 12. What molecular geometry is shown by the ethyne molecule, C2H2 ? A. linear B. trigonal planar C. tetrahedral D. bent 13. Which of the following molecules displays a bond angle close to 109.5o ? A. C2H4 B. NH3 C. BF3 D. CO2 Answer Key 1. 2. 3. 4. 5. A D C D B 6. D 7. B 8. A 9. C 10. D 11. B 12. A 13. B