Chapter 3: Molecular Compounds and Intermolecular Forces
advertisement
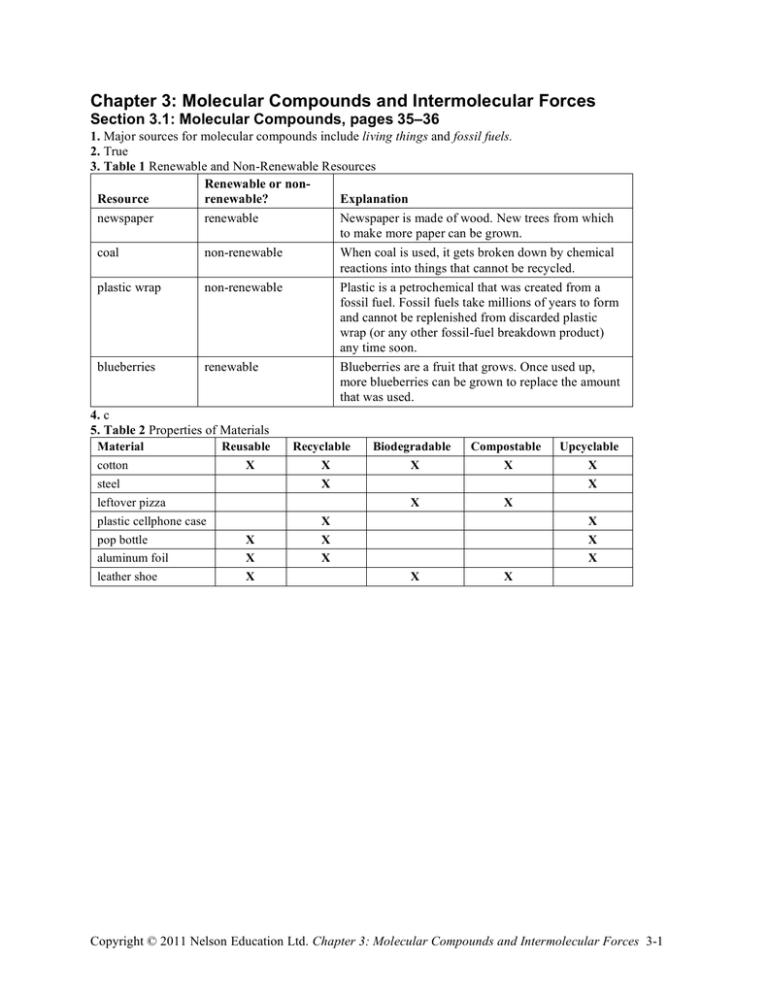
Chapter 3: Molecular Compounds and Intermolecular Forces Section 3.1: Molecular Compounds, pages 35–36 1. Major sources for molecular compounds include living things and fossil fuels. 2. True 3. Table 1 Renewable and Non-Renewable Resources Renewable or nonResource Explanation renewable? newspaper renewable coal non-renewable plastic wrap non-renewable blueberries renewable 4. c 5. Table 2 Properties of Materials Material Reusable cotton X steel leftover pizza plastic cellphone case pop bottle X aluminum foil X leather shoe X Newspaper is made of wood. New trees from which to make more paper can be grown. When coal is used, it gets broken down by chemical reactions into things that cannot be recycled. Plastic is a petrochemical that was created from a fossil fuel. Fossil fuels take millions of years to form and cannot be replenished from discarded plastic wrap (or any other fossil-fuel breakdown product) any time soon. Blueberries are a fruit that grows. Once used up, more blueberries can be grown to replace the amount that was used. Recyclable X Biodegradable Compostable Upcyclable X X X X X X X X X X X X X X X Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-1 6. Figure 1 Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-2 Section 3.2: Explore an Issue in Molecular Compounds: DEET in Insect Repellents, page 37 1. The purpose of an insect repellent is to ward off insects while the purpose of an insecticide is to kill insects. 2. Fabrics in which DEET is soluble are likely to be damaged by DEET. 3. d 4. Answers may vary. Sample answer: Table 1 Issues and Ranking for Insect Repellents Featured in a Store Issue Rank Reason the concentration of 5 This is fairly important because doctors recommend keeping the insect repellent concentration of chemicals like DEET low for children. the active ingredient in 3 This is extremely important because the active ingredient determines the insect repellent how safe and effective the product is. the price of insect 6 This is somewhat important because I will not buy the product if it is repellent too expensive. how effective the 1 This is the most important issue for me. If the product is effective, then product is I will feel good about recommending it to customers. reported health concerns 4 This is very important. If there are health problems with a given about the product product, I will not use it. how well known the 7 This is not that important. Any company is acceptable as long as its company is product is safe and effective. how safe the product is 2 This is extremely important. I will not use a product that is not safe for for children children. 5. Answers may vary. Sample answer:: I would choose a well-known brand of insect repellent that features DEET in a 10 % concentration. Any greater concentration and I think I may be at risk. Section 3.3: Polar Bonds and Polar Molecules, pages 38–40 1. In a diatomic molecule with a polar covalent bond, the electrons tend to cluster more closely to the atom that is more electronegative. 2. True 3. b 4. (a) !EN C–Cl = 3.2 – 2.6 = 0.6 !EN C–C = 2.6 – 2.6 !EN C–H =0 = 2.6 – 2.2 = 0.4 (b) C–Cl is the most polar. C–H is slightly polar. C–C is non-polar. (c) Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-3 5. Polarity of HCl: Two atoms make up HCl, one hydrogen and one chlorine. Lewis structure: The molecule has one covalent bond. !EN Cl–H = 3.2 – 2.2 = 1.0 This is polar covalent. Partial charges: With only one polar covalent bond, the molecule is polar no matter what; therefore, HCl is a polar molecule. 6. Polarity of H2: Two hydrogen atoms make up H2. Lewis structure: The molecule has one covalent bond. ΔEN = 0. This is non-polar covalent. With no polar covalent bonds, the H2 molecule is non-polar. 7. Polarity of NF3: Four atoms make up NF3, three fluorines and one nitrogen. Lewis structure: There are three covalent bonds. !EN F–N = 4.0 – 3.0 = 1.0 This is polar covalent. Partial charges: With only one polar covalent bond, the molecule would be polar no matter what. With multiple polar covalent bonds, the molecule may or may not be polar. But the molecule is asymmetrical, so it is a polar molecule. 8. Polarity of CO2: Three atoms make up CO2, two oxygens and one nitrogen. Lewis structure: There are four covalent bonds. !EN O–C = 3.4 – 2.6 = 0.8 This is polar covalent. But CO2 is a symmetrical, linear molecule, with a symmetrical placement of the two oxygen atoms on either side of the carbon, The two polar covalent bonds cancel out each other, making CO2 a non-polar molecule. Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-4 9. Polarity of NO2: Three atoms make up NO2, two oxygens and one nitrogen. Lewis structure: There are three covalent bonds. !EN O–N = 3.4 – 3.0 = 0.4 This is polar covalent. Partial charges: Since the NO2 molecule is lopsided, or bent, we can consider that it has two ends: a negatively charged oxygen end and a positively charged nitrogen end. Thus, the NO2 molecule is polar. Section 3.4: Intermolecular Forces, pages 41–42 1. Ionic compounds are held together by the ionic bonds of the elements that make up the compound, not by intermolecular forces. 2. False. A hydrogen bond is a special form of the dipole–dipole force. 3. d 4. The melting point of a molecular compound depends primarily on how tightly its molecules are held together by the three van der Waals forces. 5. Table 1 Properties of Elements Rank Type of force or bond Causes Example 1 ionic bond electrostatic force between crystalline structure of NaCl ions 2 hydrogen bond highly polar nature of small water surface tension molecules 3 dipole–dipole force polar attraction between hydrogen being attracted to nearby parts of molecules chlorine in HCl 4 London dispersion temporary gaps in electron attraction of bromine to itself in Br2 force clouds 6. (a) Ball and stick models for HCl and H2O: (b) A dipole–dipole force will arise between H and Cl in a group of HCl molecules. (c) Model of a group of HCl molecules and the intermolecular forces between them: Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-5 (d) A model of a group of water molecules and the intermolecular forces between them: 7. (a) The electronegativity difference between the two atoms in the iodine molecule is zero. (b) I2 is a non-polar molecule. (c) The explanation for why the melting and boiling points of I2 (113.7 °C and 184.3 °C) are higher than those of water, a highly polar molecule, is as follows: Iodine atoms are large—each iodine atom has 53 protons and 53 electrons. This high number of protons and electrons gives iodine atoms ample opportunity to experience London dispersion forces, which create attraction between molecules and hold the molecules together as a solid at standard temperatures. Section 3.5: Hydrogen Bonding and Water, pages 43–44 1. True 2. b 3. On a planet with an average daily temperature range of between –100 °C and –50 °C, you would expect to find (a) (i) Ammonia would be in a solid state or liquid state. (ii) Hydrogen sulfide would be in a solid state, liquid state, or gas state. (iii) Water would be in a solid state only. (b) Life as we know it operates on a planet in which a common compound exists in all three states. Therefore, of water, ammonia, and hydrogen sulfide, hydrogen sulfide would be more likely to support life on this planet. 4. (a) The water temperature in the lake might rise a degree or so, if at all. (b) Water is slow to heat up when temperatures rise. Then, when temperatures drop, water is slow to cool down. Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-6 5. Figure 1 Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-7 Section 3.6: Green Chemistry in Action: Choosing the Right Materials, pages 45– 46 1. True 2. Figure 1 3. (a) ii, iii; (b) i, ii 4. b 5. Table 1 Conventional vs. Green Products Conventional product Step requirement raw materials renewable or nonrenewable production efficient process product efficient function by-products not an issue Green product requirement renewable only Advantage of green product does not deplete natural resources efficient, every step ensures no damage to environmentally safe environment efficient without ensures no damage to environmental damage environment no environmental damage ensures no damage to environment end-ofnot an issue must have environmentally ensures no damage to product life friendly disposal environment 6. (3) Increased kinetic energy can overwhelm intermolecular forces between molecules in a plastic. (4) This can melt, deform, or decompose plastic computer cases. (6) Since they do not break down easily, BFRs cause environmental problems. (1) Computers can generate a great deal of thermal energy. (7) As an alternative to BFRs, scientists are developing bioplastics that can withstand high temperatures yet can be biodegraded. (5) To prevent damage, chemists use BFR plastics for computer cases that can withstand high temperatures. (2) Thermal energy increases kinetic energy in solid materials. 7. Answers may vary. Sample answer: I think imposing a disposal cost for disposable diapers when they are purchased would be fair. Right now, parents dispose of millions of diapers that just sit in landfills. Imposing a cost could provide funds for helping to clean these landfills, or perhaps sponsor research to develop new and better diapers. Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-8 Chapter 3 Summary, page 47 Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 3-9 Chapter 3 Questions, pages 48–49 1. d 2. (a) False. The crystalline structure of solid water takes up more space than the structure of liquid water, causing water to take up more space when it freezes. (b) False. Raindrops form as beads because of the high value of water’s surface tension that is caused by the strong hydrogen bonds that form between water molecules. 3. Table 1 Properties for Paper Towel Plastic Rank of Property importance Explanation reusable 5 This is not an important property. It is not likely that people would ever want to reuse a used paper towel. biodegradable 1 This is the most important property. Once disposed of, these paper towels need to be able to break down. compostable 2 This is important. If the paper towels are put in composts, it is worthwhile to have the plastic break down. recyclable 3 It is unlikely that people would take the trouble to recycle used paper towels, so this is not important. upcyclable 4 It is not likely that people would gather used paper towels and use them to make something. 4. (a) Behaviour of a thin stream of a compound released near a negatively charged acetate strip: (i) NF3 (ii) CH4 (iii) HI (b) NF3 and HI are both polar molecules. HI is polar because it is diatomic with two different atoms. NF3 is polar because its bonds are polar covalent and it is not symmetrical. CH4 is not polar because it is very symmetrical and its bonds are not highly polar. 5. Table 2 Unknown Compounds Compound Melting point (°C) Boiling point (°C) Force SCl2 –121 °C N2 –210 °C KCl 770 °C HF –83 °C 6. (a) biodgradable (b) function (c) fossil fuel use (d) cost (e) greenhouse emissions (f) compostable 1 5 1 5 1 1 59 °C –196 °C 1420 °C 20 °C conventional plastic conventional plastic conventional plastic conventional plastic conventional plastic conventional plastic 5 4 3 3 3 4 dipole–dipole London dispersion ionic electrostatic hydrogen bonds bioplastic bioplastic bioplastic bioplastic bioplastic bioplastic Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 310 Unit 1 Questions, pages 50–51 1. a 2. (a) Bohr–Rutherford diagram of oxygen: (b) How an oxygen atom ionizes: 3. False. The electronegativity difference between carbon and fluorine is 1.4, indicating that a C-F bond is polar covalent in character. 4. (a) The anion for the ionic compound A2(BC3)4 is BC3. The anion is polyatomic. (b) If the charge on the anion is –2, the valence of the multivalent cation is +4. 5. (a) The IUPAC name for ZnBr2 is zinc bromide. (b) The IUPAC name for Pb3P4 is lead(IV) phosphide. 6. Write the chemical formula for the following compounds. (2.4) [K/U] [T/I] (a) The chemical formula for lithium oxide is Li2 O. (b) The chemical formula for copper(II) nitride is Cu3N2. 7. Figure 2 8. (a) Water exists as a liquid at room temperature. Not special. (b) Water exists as a solid, liquid, and gas on Earth’s surface. Special. (c) Water expands when it freezes. Special. (d) Water is a common compound with a melting point of over –10 °C. Not special. (e) Water is a molecular compound with a boiling point of over 80 °C. Special. (f) Water as a solid floats on liquid water. Special. (g) Water forms droplets. Special. Copyright © 2011 Nelson Education Ltd. Chapter 3: Molecular Compounds and Intermolecular Forces 311





