Minerals - PBworks
advertisement

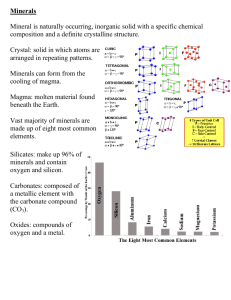
CMU Bill Palmer Lecture 4 Central Methodist University Natural Chemically Pure Make up “rocks” Solid Inorganic Often form crystals Crystal structure is organized arrangement of atoms MINERALS ARE CHEMICALLY PURE ROCKS ARE COMPOSED OF MINERALS Big important hint: Learn the Minerals and the Rocks will be easy to learn!! Your Very Life Depends on Minerals Between 2 - 3,000 have been identified A few are “native elements” -- made of only one element, such as sulfur, gold. copper, and graphite (carbon) Most are compounds, especially the silicate group (Si, O) Other important groups are oxides, carbonates, and sulfides Quartz Feldspar (group) Muscovite (white mica) Biotite (black mica) Calcite Pyroxene Olivine Amphibole (group) Magnetite, limonite, and other iron oxides Pyrite Galena Barite Aluminum--packaging, transport, building Beryllium--gemstones, fluorescent lights Copper--electric cables, wires, switches Feldspar--glass and ceramics Iron--buildings, automobiles, magnets Calcite--toothpaste, construction Hardness Crystal shape (form) Luster Color Streak Learn how to test minerals for these characteristics instead of trying to memorize each mineral!! Cleavage/fracture Density (specific gravity) Special properties --reaction to acid --fluorescence --salty taste --magnetism With 3,000 different minerals it would be next to impossible to learn all the names!! Ability to scratch another mineral Mohs scale from 1 (talc) to 10 (diamond) Quartz (most common mineral and most dust particles) is 7 HARDNESS OF SOME COMMON ITEMS: MOH’S SCALE 2.5 Fingernail 2.5–3 Gold, Silver 3 Copper Penny 4-4.5 Platinum 4-5 Iron 5.5 Knife Blade 6-7 Glass 6.5 Iron Pyrite 7+ Hardened Steel File 1 Talc Talcum powder. 2 Gypsum Plaster of Paris. Gypsum is formed when seawater evaporates from the Earth’s surface. 3 Calcite Limestone and most shells contain calcite. 4 Fluorite Fluorine in fluorite prevents tooth decay. 5 Apatite When you are hungry you have a big "appetite". 6 Orthoclase Feldspar In German, "feld" means "field". 7 Quartz Found everywhere. 8 Topaz The November birthstone. Emerald and aquamarine are varieties of beryl with a hardness of 8. 9 Corundum Sapphire and ruby are varieties of corundum. Twice as hard as topaz. 10 Diamond Used in jewelry and cutting tools. Four times as hard as corundum. External structure due to internal arrangement of the atoms Six basic groups of shapes, with about three dozen variations Describes how light reflects off the surface Main categories are “metallic” and “non-metallic” Non-metallic dull glassy waxy pearly Results from ability to absorb some wavelengths and reflect others Some minerals have characteristics colors Others vary due to chemical differences or impurities (atoms mixed inside the main elements) COLOR is usually the worst way to identify a mineral. Color of the powder when rubbed on a “streak plate” (unglazed porcelain) May be same as hand-specimen or different Some paint is based on powdered minerals (streaks) Some minerals split along flat surfaces when struck hard--this is called mineral cleavage Other minerals break unevenly along rough or curved surfaces--this is called fracture A few minerals have both cleavage and fracture Due to how the atoms are arranged Be sure you understand the difference between CLEAVAGE and FRACTURE. All minerals have density (mass / volume), but some are very dense Examples include galena, magnetite, and gold Specific Gravity is the density of the mineral compared with density of water Remember our Lab? Some minerals just feel “heavy” Carbonates react with dilute HCl and other acids by fizzing or bubbling (releasing CO2 gas) Some minerals will glow when placed under short-wave or long-wave ultraviolet rays Franklin and Ogdensburg NJ are famous for their fluorescent minerals DO NOT TASTE MOST MINERALS! Halite is the exception--it will taste salty Kaolinite (clay) will stick to your tongue Magnetite Many iron minerals will produce an invisible magnetic force field “Lodestone” was used by Vikings more than 1,000 years ago as compasses Or Some common minerals that make-up most rocks and some minerals your kids may ask you what they are and you will want to know so you can impress them. Very hard 7.0 Many, Many forms Clear Rosy=pink Milky=white Smoky=black Chalcedony=multicolor Agate=color rings Chert=white, brown, tan (very common) Mozarkite=pink/purple bands (MO State Rock) Amethyst=purple Jasper=dark blood red Jasper Amethyst Milky Mozarkite Chalcedony Smoky Agate Rosy Quartz Chert Clear Hard 6.0 Vitreous, pearly Good Cleavage, breaks at 900 Plagioclase Sodium Calcium Aluminum silicate White, Yellow, Pink Striations Orthoclase Potassium Aluminum Silicate White, yellow, pink NO Striations Check the Color ORTHOCLASE pink FELDSPAR yes light Check for Striations dark PLAGIOCLASE FELDSPAR no Soft 2.5-3.0 “Saran Wrap” Breaks in thin layers Biotite Dark Muscovite Clear Soft 3.0 Excellent cleavage in 3 directions NOT 900 Colorless White Yellowish Rhombohedrons Hard 5.5-6.0 “Augite” Dark Green to Gray Eight-sided prisms Hard 7.0 White Streak Pale to dark olive Green Peridot is the Gemstone Hard 5.5 White to gray Streak Dark gray to black Iron Oxides Iron Ores Color Varies with amount of Iron Limonite = yellow Hematite = reddish Magnetite = black Tell apart by streak Hard 6.0-6.5 Dark gray Streak Brassy yellow “Fool’s Gold” Soft 2.5 Silver Gray Metallic Perfect Cubes MO State Mineral Source of Lead Soft 3.0-3.5 White, but can be stained reddish with iron Heavy “Tiff” of S MO 1. Research the common uses for the 12 common minerals in the lecture. 2. List the diagnostic characteristics of minerals and include some of the possible variations. 3. What is the difference between a rock and a mineral? 4. Define a mineral. 5. What Moh’s Scale and what are some hardness of some common objects. 6. What is a crystal? Do all minerals form crystals? What causes a crystal? 7. What is luster? What are the main types? 8. Distinguish between cleavage and fracture. 9. Why is color a poor distinguishing characteristic when identifying minerals? 10. What is the Missouri State Mineral? www.mii.org www.mineral.galleries.com/mineral s www.mineral.net www.usgs.gov